People have leaned on potassium carbonate for centuries without realizing how often it shaped everyday life. In earlier times, folks produced it by leaching wood ashes and boiling down the water, collecting the white salt—pearl ash. Glassmakers in Venice, soap makers in England, and even bakers from Germany saw potassium carbonate as vital, whether for lowering melting points or making dough rise. By the 19th century, scientific curiosity and scaling demands encouraged chemists to improve purity and yields. Electrolytic advances and industrial processes opened the door to cleaner, reliable streams of this alkali, moving far from the open hearth to controlled factory setups. This history feels personal, reminding me of how grandparents always seemed to trust the old ways, yet, over time, new methods showed their edge and consistency.
Potassium carbonate surfaces as a white, salty-tasting powder often used in bulk for its alkali strength. It's no celebrity, but its low profile belies a resume that stretches from the foods on the dinner table to industrial glass that shields us from UV rays. This salt dissolves readily in water, feels slightly slippery to the touch, and comes in grades suited for food, pharma, or technical labs. For those on production lines, features like purity, absence of chlorides or heavy metal traces, and reliable delivery matter more than glossy packaging or branding.
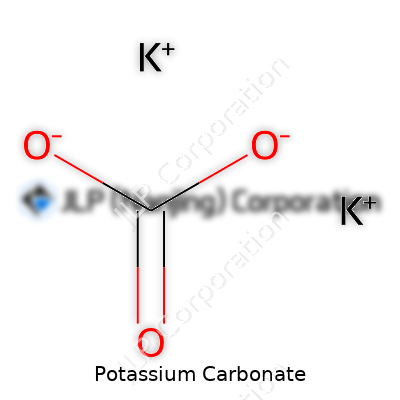
Potassium carbonate carries the formula K₂CO₃, clocking in with a molar mass of 138.21 g/mol. It stands up well in the air, won’t melt but decomposes around 891°C, and crumbles into fine particles when dry. Dump it in water and it dissolves generously, making a basic solution with a hefty pH—great if you need to neutralize acids or coax reactions forward. The solid stubbornly avoids dissolving in alcohol and feels gritty between the fingers. In my own experience handling it, the hygroscopic nature always gets me: leave a bag open in humid air, and you come back to a clumpy mess.
Product grades reflect use-cases—industrial, food, analytical—and the right label spells out purity levels, presence of sulfates, chlorides, loss on drying, and heavy metal content. For food use, top players guarantee grades free from hazardous traces, confirmed by third-party labs. Europe and North America both regulate labeling, so you encounter batch numbers, date of production, and recommended storage to guide safe handling. Lab techs and plant workers alike glance at those stickers for key info, knowing that even a tiny impurity can derail a day's work.
Modern manufacturers often rely on potassium hydroxide reacting with carbon dioxide, either bubbling gas through a potassium hydroxide solution or carbonating potassium-containing raw materials. The solution gets filtered, evaporated, and dried, leaving behind the glassy, white powder. This method delivers better yields than the old boiling-ash routine and sidesteps many contaminants. I've watched plant operators check for crusts or off-colors, which might point to raw material changes or hiccups upstream. The shift to closed-loop systems did wonders for cutting down on both waste and energy use.
With a backbone as a basic salt, potassium carbonate acts as a launchpad in all sorts of reactions. It neutralizes acids, precipitates metals, or acts as a drying agent in organic synthesis. React with hydrochloric acid, and you get potassium chloride plus fizzing CO₂. Heat it hard and it releases carbon dioxide, leaving potassium oxide. Scientists in R&D tweak it into mixes, like double salts or complex oxides, hunting for catalysts or water-softening blends. I've seen students thrill at watching it bubble in flasks, the certainty of a reaction visible in the hiss and cloud of escaping gas.
This alkali goes by many aliases: potash, pearl ash, carbonate of potash, and E501 when dropped into food applications. Chemists reach for K₂CO₃ while food manufacturers check for E numbers. In the trade, folks will say 'potash' and mean a whole world of potassium compounds, so you need to double-check actual content and specs before use. Warehouses track skip loads marked with every name in the book, sometimes causing confusion among newcomers in the industry.
On the factory floor, safety protocols stand at the front. Potassium carbonate’s alkaline bite can irritate skin and eyes, so protective gloves and goggles come as standard. Spill kits and eye-wash stations offer backup for the inevitable splash. Industrial operations set limits on airborne dust and require proper storage in sealed bins to keep clumping and hydration at bay. Training drills focus on spill containment and quick cleanup, because small accidents can become bigger ones fast if the powder soaks up moisture and hardens on surfaces. Regulations from OSHA and REACH require documentation for every shipment and track worker exposure over time.
You find potassium carbonate tucked into an impressive lineup of applications. Glassmakers rely on it to drive clarity and reduce melting points. Soap producers favor it for the mild, creamy lather it brings—unlike more aggressive sodium-based alternatives. Winemakers and cocoa processors adjust acidity with this salt, while Chinese noodle makers cherish its role in making chewy alkaline dough. Laboratories put it to work as a drying agent, and water treatment plants deploy it to soften municipal supplies. Battery and fertilizer industries need potassium carbonate for chemical synthesis and soil amendment. Juggling these uses, industries count on both the reliability and applications that really get the job done—one batch at a time.
Researchers extend potassium carbonate’s value chain by investigating how tweaking production influences performance and environmental impact. Teams study new sources of potassium, aiming to cut dependence on mines while lowering energy footprints. I recall reading journals on using agricultural waste as a raw material—turning what farmers once burned or buried into industrial feedstock. Scientists in materials engineering test modified potassium carbonates for better conductivity or compatibility in batteries. Biochemists look at using it in enzyme reactions or greener extraction processes, hoping to thread sustainability into every batch. R&D also circles back to safety, examining ways to minimize health risks or develop smarter packaging that withstands evolving global regulations.
Toxicologists chart potassium carbonate’s effects through acute and chronic exposure studies. While it carries lower toxicity than many industrial salts, concentrated solutions burn mucous membranes and irritate lungs if inhaled. Over the years, doctors tracked cases of skin rashes or accidental ingestion, mostly among workers or children. Strict workplace limits and public education prevent the kind of mishaps that once plagued unregulated workshops. Research continues to fine-tune concentration thresholds for food and water, ensuring that trace levels help, not harm. Animal studies and clinical reviews watch for long-term impacts, making sure our appetite for processed foods doesn’t come with unseen risks.
Looking ahead, potassium carbonate seems poised for a quiet growth spurt. As demands for renewable energy and lightweight glass climb, producers keep upgrading processes to hit tighter specs and sustainability goals. Smart recycling and recovery—using waste streams to reclaim potassium—could keep supply steady without leaning so hard on mining. In battery production, tweaks in purity might help jump-start next-gen energy storage. New food uses are under study, especially as societies hunt down preservatives gentler on body and planet alike. Environmental watchdogs will likely push tighter controls, pushing manufacturers toward better stewardship with each passing year. Whether in innovation or regulation, potassium carbonate will keep shaping products we count on—without ever clamoring for the spotlight.
Potassium carbonate might sound like something you’d only find in a laboratory, but regular folks use it, or at least benefit from it, more often than they realize. It helps make soap work the way people expect. Grab an old-school bar of handmade soap and you’ll usually find potassium carbonate on the ingredient list. It lets the soap lather up right, even in hard water, turning what would be a sticky mess into something that actually feels clean. That alone makes everyday cleaning easier.
On farms and in gardens, potassium carbonate brings another benefit. Plants need potassium to grow strong, so farmers and hobby gardeners mix this white powder into fertilizer blends. Tomatoes, potatoes, and grape vines all crave it. Without it, crops droop, fruit shrivels, and yields collapse. Boosting soil with potassium carbonate helps create bumper crops and healthier produce on the dinner table.
Potassium carbonate has earned a place inside the kitchen, too. Bakers add it to gingerbread and certain noodles to control texture and color. In Chinese cuisine, it goes into mooncakes and ramen noodles. The compound shifts pH to boost flavors and bring the right chewiness to noodles that turn a simple meal into something special.
Pretzels and some European cookies get their signature look and taste after a bath in a potassium carbonate solution—think of that brown, glossy crust. Without it, the color and the bite would both feel off. Food workers trust the rules: the compound needs careful handling, and keeping the right dosage matters for safety and taste.
Brewers and winemakers keep potassium carbonate around to adjust acidity or "soften" water. Sour grapes or over-acidic mash spoil a whole batch of wine or beer, so this simple powder keeps drinks tasting right and customers happy.
Glassmakers, too, owe a lot to potassium carbonate. Mixed with sand and other salts, it raises the melting point and helps control the clarity and finish of both fine crystal and everyday drinking glasses. Anyone drinking from a sparkling tumbler owes a quiet thanks to potassium carbonate.
Laundry used to be a back-breaking job before modern detergents hit the shelves. Potassium carbonate is an old-school cleaning power ingredient, working to break up stains and soften water years before synthetic detergents came along.
Some will say that seeing potassium carbonate listed on a product is no big deal. Walk through a grocery store and people look right past ingredient lists, assuming everything is safe and needed. But it pays to look closer. Chemicals like potassium carbonate fall under tough safety standards. Industry folks keep careful track of how much gets used and monitor for any risks. Also, waste from factories using potassium carbonate can affect rivers and land if companies cut corners, so holding manufacturers to account matters for all of us.
Looking at how a simple white powder can help a tomato plant, a loaf of bread, a glass of wine, and a sparkling window says a lot about how science quietly improves daily living. What matters most is that people stay curious and keep asking about what’s in the products they buy and use. That sort of awareness gets better results—not just in taste or cleaning but in caring for the land and health.
Most people won’t come across potassium carbonate outside a lab, a factory, or the odd specialty cleaning product. But for anyone working in these places, the white, powdery substance gets a spot among other well-known chemicals. Potassium carbonate crops up in everything from soap making to winemaking and even some food processing. The stuff doesn’t come with a dramatic warning. Still, it deserves real respect.
Potassium carbonate doesn’t behave like bleach or strong acids, but it’s no harmless kitchen powder. Touching it with bare hands can dry out skin or cause irritation. Accidentally breathing in the dust might sting in the throat or set off a cough. Add water, and it starts to slip more into the “alkali” territory—able to react with acids, even strong enough at certain strengths to nibble away at tissue. My own experience with basic chemistry tells me: you don’t ignore what you can’t immediately see. A small cloud of dust, especially indoors, creates havoc quickly, and rinsing your hands carefully gets messy if you don’t pay attention.
Jobs involving raw chemistry still end up shaped by habits, laziness, and everyday mistakes. One day, someone forgets to snap on gloves, convinced “it’s just a quick task.” Over time, small exposures add up—a mild rash, rough skin, or even reddened eyes after rubbing them. Handling potassium carbonate safely isn’t about paranoia. It’s about remembering that small, visible steps—dust masks, gloves, eye protection, keeping containers sealed—protect more than just skin. They protect your routine, your workspace, and the people around you. Once, an old coworker decided to “brave it” and forgo gloves. By the end of the shift, he had tight, dry patches on both hands. All that could have been avoided by a simple layer of nitrile.
Look up potassium carbonate’s Material Safety Data Sheet, and you’ll find skin irritation, serious eye irritation risks, and respiratory effects from dust. The numbers aren’t wildly scary, but the trends add up. Statistically, most injuries in chemical work don’t come from one-off disasters but from sloppy routines and boredom. Over time, folks stop paying attention, especially with a substance that's labeled “mildly irritating” instead of “danger: fatal.” The danger ramps up if you combine it with acids or don’t clean up spills, as heat and fumes may form. It’s a low-level hazard, but one that never quite goes away or announces itself with a bang.
Changes don’t require expensive overhauls. Workers need solid gloves, protective goggles, and ready access to running water. These things don’t cost much or slow down a job. Storing chemicals right, labeling everything clearly, and running regular reminders about safety work wonders. One manager I worked with built a “no exception” rule for PPE. He wasn’t trying to scare people—he just didn’t want to explain skin burns or eye washes to family members later. Simple checklists and firm habits reduce trips to the sink, bandages, and awkward conversations with health officers.
People sometimes look to rules, training videos, or company memos as the main forms of chemical safety. In reality, it comes down to what happens on a regular Tuesday afternoon. Personal responsibility—not just for yourself, but for coworkers—matters more than any official guide. Spend a moment to suit up, check containers, and remind those around you to do the same. In the end, these quiet, steady actions—more than warnings or scary stories—keep people healthy and out of the emergency room.
I’ve learned that handling chemicals like potassium carbonate isn’t only about what’s on a label. At home and at work, small lapses in storage can turn into big headaches. Anyone dealing with this compound, whether it’s in the glass industry or for soap making, knows it reacts with moisture. Leave the lid off a container on a humid day, and pretty soon you’ll come back to a chunky, caked-up mess. Water in the air clings to potassium carbonate like glue. Suddenly, a dry powder turns into a syrupy paste — at that point, you have waste on your hands and an unnecessary expense to replace it.
I grew up in a rural area where folks tended to store everything in old coffee cans and jars. That habit doesn’t work for chemicals like this. Potassium carbonate pulls in water so aggressively, plastic bags and the wrong type of jars become a problem. Regular people, not just safety managers, benefit from sealed, air-tight containers. Not all plastics are up for the job — certain plastics break down over time, which means you can end up with your powder all over the bench or worse, mixed with material it wasn’t supposed to contact. Using metal containers turns out even worse. Old coffee cans or anything that isn't specially lined can rust, and moisture plus potassium carbonate can turn lids into a science experiment no one wants to clean up.
The physical space matters just as much as the container. Kitchens, basements, or sheds sometimes flood or stay muggy through the summer. High humidity speeds up the absorption of water, so choosing a dry, well-aired room makes a real difference. One easy trick I use is stashing a desiccant pouch inside the container, especially in the type of dusty workshops and garages where other supplies might sit. If you don’t have big industrial dehumidifiers, just keeping things off the ground helps avoid dampness seeping up from below.
Letting potassium carbonate absorb water isn’t only a problem for its shelf life. Spilled or damp powder turns slippery. I’ve watched someone learn this the hard way — one slip and suddenly there’s a back injury in the making. The chemical shouldn’t be anywhere near acids or strong oxidizers like bleach. Mixing those by accident, even in trace amounts left in reused containers, can release gas or heat, risking burns or respiratory trouble. Keeping all chemicals clearly labeled and away from incompatible substances keeps workspaces safer.
Organization matters. Without a storage plan, chemicals wander from one end of the shop or pantry to the other. Once, I found a packet stuffed into a drawer near a leaky window. It had swollen up, stuck itself to the wood, and left a white crust all over. It took more time and effort to clean up than if someone had put it in a sturdy, labeled tub on a high shelf. Clear, sturdy, dry, and sealed — those four rules avoid most headaches.
Plenty of solutions don’t require special training. Clean spoons or scoops can reduce contamination if they’re dry and set aside for one use. For households or small workshops, even a basic plastic storage box with a gasket and some silica gel packs outperforms a fancy chemical cabinet if you’re on a budget. Good habits, simple tools, and a couple of basic precautions keep potassium carbonate useful and safe until the last scoop.
So, potassium carbonate. Chemically, it carries the formula K2CO3. That combination of two potassium atoms, a carbon atom, and three oxygen atoms really means something far beyond just symbols on a label. Potassium pops up in soil, bananas, and the fertilizer aisle at any garden center, but potassium carbonate takes a different path. People in glassmaking, soap production, and even brewing have been using it since well before most of us even learned the periodic table.
Potassium carbonate manages to show up in surprising corners of everyday life. Think about making noodles, softening hard water, or keeping chocolate silky-smooth. K2CO3 plays a part in all of that. Potash, one of its older names, helped families leach ashes into soap and glass for years before industrial chemistry changed the recipe books. Every time I’ve cleaned a stained coffee cup at home, it’s something like this cleaning magic working in the background.
A lot of people probably don’t realize potassium carbonate slides into food production. European licorice gets its color and bite thanks to this compound. Beyond food, it acts as a buffer against the corrosive nature of acids, helping us keep boilers and piping systems healthy. In greenhouses, I’ve watched commercial growers use it to push potassium levels higher in hydroponic tomatoes. More potassium often sparks stronger growth, bigger fruit, and better taste.
Chemists produce K2CO3 by reacting potassium hydroxide with carbon dioxide, releasing nothing more dangerous than water as a by-product. This is a far cry from older processes where wood ash meant sooty kitchens and a fair bit of trial and error.
No chemical comes without hiccups. Potassium carbonate works wonders for cleaning, but it’s strong stuff. Left unguarded, young kids could end up touching or tasting it, which leads to burns and breathing problems. Just because something is useful in agriculture or baking doesn’t mean it suits every home with no questions asked. I keep my bulk garden supplies well out of reach, and warnings on packaging point out the risks, but not everyone reads those.
Like many fertilizers, overapplication brings runoff problems. Extra potassium, washed into waterways, pushes ecosystems out of balance. Water treatment plants may struggle to keep up, and algae blooms take over. Instead of backing away from using potassium carbonate, there’s room for better training — both on the farm and in urban gardens. Label clarity, more chemistry lessons in schools, and a culture that values safe handling can help curb these issues. Industries could support community recycling of chemical containers to keep empty bottles from building up in backyards.
People often talk about chemicals as if they’re only trouble in powder form or sealed drums. Truth is, K2CO3 makes daily life a little easier, whether that’s crispier pretzels, clear glass, or better-tasting vegetables. The more everyone understands the formula and its fingerprints on the world around us, the smarter choices we can make. I’ve learned to respect both the benefits and the hazards by seeing how a little knowledge can turn kitchen or garden experiments into something rewarding.
Potassium carbonate absolutely dissolves in water. We’re not talking about a little bit here and there—it breaks apart quite easily. Toss it into a glass, give it a stir, and you end up with a clear solution. No gritty chunks at the bottom, no floating bits. Here’s the science: potassium carbonate, with the formula K2CO3, pulls apart because the positive potassium ions and the carbonate ions both love hanging out with water molecules. You basically can’t stop this stuff from vanishing into solution unless you really jack up the concentration.
I spent some college years working with chemistry kits, figuring out why some compounds just wouldn’t budge in water. Potassium carbonate made life easier because it disappeared so quickly. You don’t have to fight with it the way you fight with some stubborn salts. This property turns a boring fact into a useful tool. If you want to alter the pH of something bland like water or a soft drink, potassium carbonate steps in. For folks in food science, brewing, even the guy who’s pickling cucumbers at home, knowing which salt mixes completely makes the process predictable and controllable.
Take food processing. Bakers used potassium carbonate for centuries, especially in old-school recipes for gingerbread. That tradition started long before modern chemical know-how; cooks just noticed that dough mixed better and rose higher with this white powder. Wash it away in water, and you get a reliably alkaline solution—super handy when you want a certain texture or taste.
Brewers often reach for potassium carbonate because it regulates acidity and improves flavor. If the water comes with too much acid, beer can taste off—flat or harsh. By dissolving potassium carbonate in the brewing water, they keep things balanced. This isn’t about following fads; users learned what works through experience, sticking with simple tricks that just keep delivering.
The easy solubility sounds like an open invitation, but that comes with a catch: overdo it, and you’ll really tip the pH scale. Dump in too much potassium carbonate and you suddenly have an environment that can ruin recipes or hurt plants. A garden I helped with last summer hit this wall. The guy wanted to fix acidic soil and thought, “More is better.” After one rainy week, the plants looked worse, roots damaged by all that alkalinity. The lesson runs clear—solubility helps with mixing, but careful measurement is non-negotiable.
Besides gardening mistakes, there’s a bigger question around safety. Potassium carbonate’s solutions get quite basic, even caustic. In factories, workers stay protected with gloves and goggles, and schools keep it out of reach for good reason. It deserves respect, just like bleach or lye, and proper handling goes a long way toward avoiding accidents.
Chemistry classes drill home basics about dissolving salts, but practical experience still holds the trump card. Teachers should get their students involved in hands-on mixing. A clear demonstration—stirring potassium carbonate into water, checking the pH, testing its impact on plant cuttings—beats any textbook. Smart labeling in packaging also helps, with quick guidelines for how much to use in cooking, gardening, or brewing. That way, people get the benefit without running into trouble, all courtesy of this highly soluble powder and a little common sense.
| Names | |
| Preferred IUPAC name | Potassium carbonate |
| Other names |
Potash Pearl ash Carbonate of potash Dipotassium carbonate Potassa |
| Pronunciation | /poʊˈtæsiəm ˈkɑːrbəneɪt/ |
| Identifiers | |
| CAS Number | 584-08-7 |
| Beilstein Reference | 358726 |
| ChEBI | CHEBI:131526 |
| ChEMBL | CHEMBL1201767 |
| ChemSpider | 5649 |
| DrugBank | DB11095 |
| ECHA InfoCard | ECHA InfoCard: 030000003916 |
| EC Number | 209-529-3 |
| Gmelin Reference | Gmelin Reference: **1847** |
| KEGG | C01304 |
| MeSH | D017746 |
| PubChem CID | 103556 |
| RTECS number | TS7750000 |
| UNII | 1DVB4A4JVY |
| UN number | UN number: "UN 1813 |
| Properties | |
| Chemical formula | K2CO3 |
| Molar mass | 138.205 g/mol |
| Appearance | White, odorless, hygroscopic crystalline powder or granules |
| Odor | Odorless |
| Density | 2.43 g/cm³ |
| Solubility in water | 1120 g/L (20 °C) |
| log P | -2.0 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 10.33 |
| Basicity (pKb) | pKb ≈ 3.7 |
| Magnetic susceptibility (χ) | −63.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.427 |
| Dipole moment | 0 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 120.1 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1151.1 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1151.0 kJ/mol |
| Pharmacology | |
| ATC code | A12BA01 |
| Hazards | |
| Main hazards | Irritating to eyes, respiratory system and skin |
| GHS labelling | GHS07, GHS hazard statements: H319 |
| Pictograms | GHS07,GHS08 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | P264, P270, P280, P301+P312, P330, P305+P351+P338, P304+P340, P312, P337+P313, P501 |
| NFPA 704 (fire diamond) | 2-0-1 |
| Lethal dose or concentration | LD50 (oral, rat): 1870 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 1870 mg/kg |
| NIOSH | RN887 |
| PEL (Permissible) | 15 mg/m3 |
| REL (Recommended) | 0.003 mg/m³ |
| Related compounds | |
| Related compounds |
Potassium bicarbonate Sodium carbonate Potassium chloride Potassium hydroxide |