Nonylphenol didn’t just show up overnight. Its roots stretch back to the early 20th century, spun out of the synthetic chemistry boom that shaped so many chemicals used today. Big demand for detergents and surfactants pulsed through post-war economies in the 1950s and 60s, opening the door for nonylphenol as a key player in making things clean, slick, or mixed. Production rolled up over years, piggybacking on the widespread switch to industrial detergents in factories, food processing plants, and even households. Modern regulations started trailing this growth decades later, only after evidence connected the dots between persistent chemical footprints in water and health impacts downstream.
Nonylphenol usually shows up as a pale yellow liquid, one that sticks around in lots of places where soaps or cleaning agents get made. Anybody who’s worked in paint, agricultural chemicals, or textile mills might have heard the name. It plays the role of both a base chemical for more complex surfactants and an intermediate in many industrial syntheses. Its appeal runs on the back of its effectiveness breaking down oils and fats or just making stuff mix where it otherwise wouldn’t.
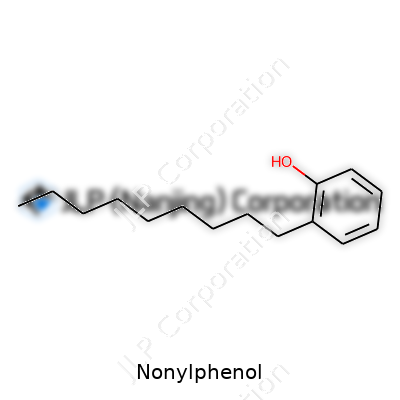
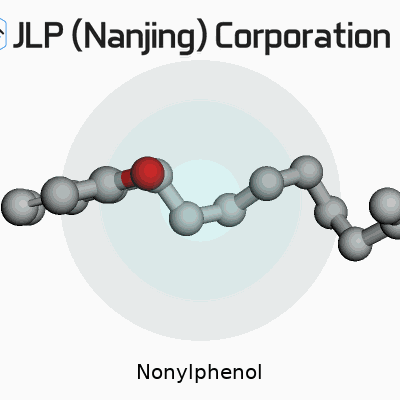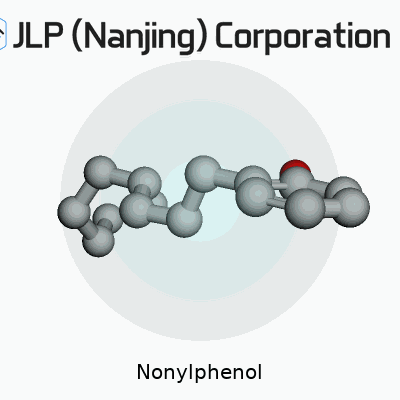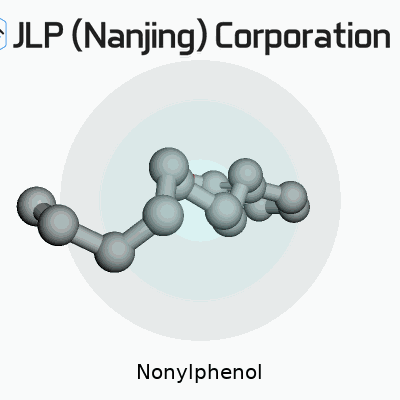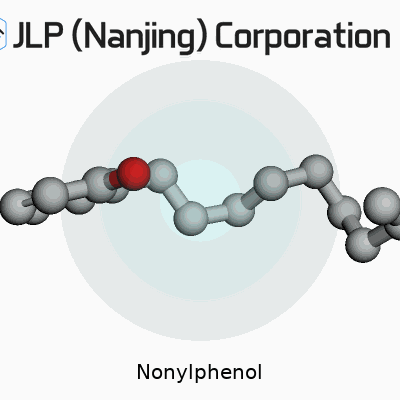
What sets nonylphenol apart is its stubborn resistance to easy breakdown. Structurally, it’s built from a phenolic ring tacked to a nine-carbon (nonyl) chain on one end, which gives it a mix of oily and polar behavior. Melt point lands around 7–10°C, with a boiling point near 295°C, telling you just how stable it remains across a wide temperature range. Runny at room temperature, it likes latching onto fat and doesn’t dissolve much in water—these traits explain both its industrial value and its persistence in the environment. Reactivity points mainly to the phenolic group, which can form ethers or esters or undergo further substitutions.
Suppliers peg purity anywhere from 90% up, usually listing both the para- and ortho-isomers by weight; the para one tends to take up the bulk. Labs and factories get supplied under strict guidelines around labeling, often with hazard symbols tied to toxicity or aquatic impact. Common catalog numbers differ company to company, but handling instructions always line up: keep containers closed, shield skin and eyes, and avoid open drains. Industrial sheets flag its chemical identity as C15H24O.
Making nonylphenol starts off simple, at least on paper: catalyze a Friedel–Crafts alkylation, where phenol reacts with nonene under acid catalysis, typically using an aluminum chloride or acidic resin catalyst. The reaction spits out a blend of isomers, but the para form shows up most. Scale and rigor boost costs, since leftover catalysts and harsh reaction conditions demand tight control over byproducts. Years ago, many chemical plants dumped washwater straight out, but tighter environmental watch now forces capture and treatment steps.
People don’t really buy nonylphenol for itself—they want what comes after. Add ethylene oxide (often in batches of 4-20 moles), and you get nonylphenol ethoxylates, those workhorse surfactants sitting in shampoos, laundry powder, weedkillers, and even some plastics. Nonylphenol undergoes esterification, sulfonation, and halogenation—jobs that switch up how it interacts with water, dirt, or biological tissue. It’s the backbone for a surprising number of formulas where blending oil and water is the headache.
Chemical catalogs list nonylphenol under names like para-nonylphenol, 4-nonylphenol, or NPh. Technical bulletins from different countries, or old manuals, sometimes just call it NP. Once it got rolled into complex surfactant mixtures, labels might only mention the ethoxylate numbers—NP-10, NP-20, and so on—pointing to the number of ethylene oxide units tacked on. This array of names creates plenty of confusion, especially in low-regulation or cross-border trade.
Anyone who’s spent time in a plant handling nonylphenol knows the drill: gloves, goggles, lot of fresh air, and tight waste controls. Breathing in vapors or letting it soak the skin isn’t just uncomfortable—it links to acute irritation and longer-term health risks. Spill drills get drilled into staff for a reason, since nonylphenol’s stubbornness means once it hits soil or water, it doesn’t just disappear. Plant operators follow strict disposal rules, bundling up even trace residues into hazardous waste streams. Regulations in the EU and North America keep getting tighter. REACH in Europe and EPA rules in the US clamp down on emissions, disposal, and allowable use in consumer products, all sparked from mounting toxicity evidence.
Industries stake a lot on this molecule. Walk through a modern textile mill or pesticide plant, and you’ll cross paths with nonylphenol somewhere along the process. Paints, adhesives, emulsifiers, even lubricating oils—all draw on its powerful surfactant-derived properties. Textile finishing, leather curing, and even certain plastics work it into their formulas. Some degreasers and cleaners still leverage its power, even as greener alternatives start to catch up. Wastewater treatment and paper production also count on derivatives for stubborn dirt removal or pulp processing, though replacements get phased in where laws demand.
Labs worldwide keep chasing ways to curb nonylphenol’s persistent fallout. Biologists, chemists, even engineers dig into alternative alkylphenols or biobased surfactants to break the industry’s long-term reliance. Advances in bioremediation show promise—isolating bacteria or fungi that chop up nonylphenol faster than anything found in the wild. Instrument makers refine detection technology, pushing environmental monitoring to catch minute traces across everything from river sediment to polar ice. Collaborations between universities and companies sometimes struggle with cost, since new surfactants might bump up prices or take years to certify for broad, safe use.
If there’s one reason regulators keep chasing this molecule, it comes down to toxicity. Decades of animal studies point to hormone disruption—nonylphenol mimics estrogen, interfering with fish, amphibians, even mammal reproductive cycles. Waterways near old plants sometimes record skewed sex ratios or unusual mutations in local wildlife. Mounting evidence also draws links to potential human health impacts, especially with chronic, low-dose exposure through food or drinking water. Authorities in Europe pushed to list nonylphenol as a substance of very high concern, and this flagged its derivatives for phase-outs in many product categories. Legacy contamination sticks around, pushing researchers to keep tracking both short-term poisoning and longer reproductive or developmental impacts.
Nobody in the chemical business thinks nonylphenol will vanish overnight—not with so many industrial chains still dependent. That said, demand keeps tilting away from its use. Companies pour funds into greener surfactants, some leaning on sugar chemistry, others turning to new alkoxylates that break down faster. Tougher waste treatment and process upgrades cut emissions, but cost and technical inertia can slow the shift. Eco-labels and consumer watchdog groups play a growing role, especially as public awareness about micro-pollutants rises. Policy and regulation keep tightening the vise, meaning the future likely holds less nonylphenol, not more. Industry looks to phase it down, either by finding new formulations or stretching out compliance for hard-to-replace applications. On the other side, universities and startups keep hunting for a drop-in replacement that packs the same punch without sticking around for decades in the water, soil, and food chain.
Nonylphenol has a way of showing up where you least expect it. You wash your dishes, clean your clothes, maybe even walk across a freshly mopped floor—you’ve probably come in contact with products touched by this chemical. It works as a powerhouse surfactant, helping detergents break up grease and grime. On paper, that looks like progress; the job gets done, and the stains are gone. But that’s just the top layer of the story.
Factories keep nonylphenol close at hand for more than household cleaners. Textile producers lean on it to process and dye fabrics so colors set evenly. Pulp and paper mills put it to work breaking down nasty stuff in recycled fibers. Paint companies mix it in to help pigments spread smoothly. It’s hard to ignore how widely this compound threads through industrial processes.
Agriculture claims a piece of the pie, too. Some pesticides use nonylphenol-based compounds to stick better to plant leaves, hoping for better pest control. Plastics manufacturers rely on it to give their products desired qualities—flexibility here, durability there. This behind-the-scenes role makes nonylphenol seem indispensable to certain sectors, despite the growing pushback.
The story gets complicated fast. Nonylphenol doesn’t break down easily once it escapes down the drain. Water treatment plants can only do so much before it slips past into rivers and lakes. That’s where ecosystems take the hit. Fish and other aquatic life face hormonal disruptions, since nonylphenol acts a lot like estrogen once inside a living body. If you’ve read reports about odd changes in fish populations—males with eggs, odd behaviors—nonylphenol often sits among the suspects.
This reach doesn’t just matter to creatures underwater. Traces linger in the food chain because nonylphenol builds up in fat tissue, working its way from tiny organisms up to larger predators, and even humans. It’s hard to put that out of mind, especially knowing that the chemical can mimic hormones, stirring up worries about fertility and child development over time.
Environmental groups and regulators have not sat this one out. The European Union moved to restrict nonylphenol—turning the spotlight on substitutes that offer cleaning or manufacturing power without the long-term fallout. The United States started banning its use in some cleaning products and put tighter rules on industrial runoff.
Newer, “greener” surfactants have made their debut, showing that companies can get results without throwing ecosystems under the bus. Some manufacturers have overhauled their recipes, turning to these alternatives not just to dodge regulations but as a selling point for eco-minded customers. My own experience switching to “green” household products taught me to look past shiny ads and dig for brands that post clear chemical lists—and the difference in conscience, if not always in performance, sticks with me.
Consumers wield power at the checkout aisle. Choosing products labeled “nonylphenol-free” sends a signal upstream to producers who face the decision every day: stick with business as usual, or chart a new path. Laws push from one side, people push from the other—leaving nonylphenol caught in the crossfire. The point is, actions—big or small—carry weight. Cleaner rivers and healthier communities are built product by product, choice by choice.
Walking down the detergent aisle or using certain cleaning agents, most people don’t think twice about what’s inside the bottle. Nonylphenol, tucked deep in the ingredient list, hits closer to home than most folks realize. Used in everything from laundry soaps to some plastics, this chemical pops up in places you wouldn’t expect. Talk around industrial circles sometimes brushes off these ingredients, but peer into the research and a different story starts to surface.
Picking up laundry pods or all-purpose cleaners, I rarely consider their ripple effects beyond sparkling countertops or fresh-smelling bedsheets. Science keeps reminding us there’s more to the story. Nonylphenol can mimic hormones in the human body. Lab studies have spotted it influencing the reproductive systems of animals, messing with hormones, and, at times, causing developmental troubles. While many studies target fish or rodents, these findings set off alarm bells about what unchecked exposure could mean for people.
Looking at neighborhoods close to certain factories, community health groups have noticed more cases of fertility issues and hormonal imbalances. This points to a connection worth serious attention—especially for those who work directly around these substances or folks living near discharge sites.
Take a look at any stream near a factory or landfill, and the odds are higher now that you’ll find traces of nonylphenol. Unlike some chemicals that break apart after short exposure to sun or water, this one hangs around. Fish and other water life are particularly vulnerable. In several rivers around the world, research shows stunted growth or odd mating patterns among aquatic animals, all linked back to elevated nonylphenol levels.
Excess chemicals slipping past outdated water treatment plants end up in drinking water and irrigation systems. Over time, this builds up in river sediments and even the food chain. Local fishermen report catching fish that seem “off,” a wake-up call that contamination doesn’t just pass by unnoticed.
Most of us don’t see riverbanks or marshes as extensions of our kitchen sink, but the chemicals we rinse out eventually make their way back around. There’s a real, direct trail from under the bathroom sink to the wider ecosystem.
Finding nonylphenol-free products takes a sharp eye and some legwork. Some brands have started booting the chemical from their recipes, but it can feel like decoding an alien language to keep up with all the labels and technical jargon at the store. Government bans in the European Union pushed industries to rethink their chemical laundry lists. But in other places, regulation moves at a crawl, with voluntary swaps for safer ingredients lagging behind the science.
Communities near industrial discharge sites often band together, pushing for environmental testing and modern water filtration. Building better sewage infrastructure doesn’t happen overnight, but a handful of committed cities now screen and cut out harmful chemicals like nonylphenol. Change often comes from the ground up: parents demanding clean playgrounds, farmers asking for chemical-free irrigation, or workers pressing for safer conditions on the job.
Raising awareness becomes a powerful tool. I’ve seen small towns hold workshops teaching folks how to read product labels or switch to old-fashioned cleaning hacks. These everyday steps help chip away at the problem, especially as people realize the most powerful vote happens on every shopping trip. The hope rests on both tighter government standards and regular people working together for healthier lives and cleaner rivers.
Nonylphenol isn’t something you want to handle without a clear plan. Most folks bump into this chemical in factories where detergents, plastics, or paints are on the menu for the day. Nonylphenol’s got a reputation for throwing trouble at hormones and messing with water life. Anyone who’s spent time around drums or vats of the stuff knows it has a way of hanging around in places it shouldn’t.
If you’re heading into a shift that involves nonylphenol, there’s no glory in working bare-handed or skipping the goggles. I learned the hard way years ago—just a splash on my skin left a nasty rash for days. Always reach for gloves (nitrile beats latex here), sleeves, and safety glasses or a face shield. Steel-toed boots aren’t just about dropped tools; nonylphenol eats through cheap footwear.
An open window doesn’t cut it. You want real air flow over the workbench. At one chemical plant I visited, a worker cracked a door to keep fumes down—ended up spreading the stink through the whole building instead. Local exhaust fans keep vapors away from your face and out the door, not recirculated through the AC. If you smell something sharp, you’re breathing more than you should. Respirators aren’t just for show—get yours fit-tested, and swap filters often.
Spills don’t wait for a convenient time. If nonylphenol hits the floor, it spreads like syrup and sticks around. Always keep spill kits within arm’s reach—absorbent pads, neutralizing agents, tough bags. Bystanders need to step out, and cleanup crews should not cut corners. Years ago, one worker used a mop and bucket, spread the mess wider, and trashed his mop. Good spill kits save everyone a lot of grief.
Nonylphenol eats through flimsy drum seals and doesn’t care for sunlight or hot temperatures. Never leave it next to acids, bases, or anything flammable. Put the drums on racks, not bare concrete, and slap on labels so nobody gets lazy with the sorting. Double-check shelf life, and log every drop you move—the worst surprise is a leaking container someone forgot to report.
No matter how many times you go through safety training, someone always tries to cut a corner or rush a process if the foreman isn’t watching. Frequent training isn’t just a formality. Get everyone familiar with the symptoms of exposure—headaches, skin burns, eye irritation. Have emergency showers and eyewash stations serviced and marked. Walk the spill route once a month, and drill your team so muscle memory kicks in, not panic, if something spills or splashes.
Down the drain? Forget it. Nonylphenol sticks around in the water supply. Grow-ups deal with disposal through certified chemical waste handlers. Keep each bottle tracked—every ounce out the door needs a record. I once had a regulator demand every page for three months’ worth—being organized meant no fines or headaches.
Staying safe with nonylphenol boils down to knowing your gear, trusting your senses, and refusing to shortcut the system. Teams who learn from old mistakes, keep their heads up, and fix risk before something goes wrong tend to get home safely. Rules and policies matter a lot, but folks looking out for each other matter more.
If you’ve ever had to deal with chemicals like Nonylphenol, you start to understand that tossing them on a shelf or into a bin is not just risky, it can end up costing your health. The pungent smell is one thing, but the real trouble comes with long-term exposure. Imagine working in a cramped storeroom where someone’s idea of “proper storage” means a barely labeled drum shoved in a corner—that’s when leaks, spills, and contamination like to turn up.
Nonylphenol, mostly used in cleaning products and industrial processes, hangs around in the environment. This stuff doesn’t just vanish after use. Touch or inhale enough, and your body starts pushing back. Once, I saw a warehouse manager brush off a sweating drum as “just a bit of humidity.” Two days later, that spot was cordoned off for cleanup, and people got headaches just from walking by.
Holding Nonylphenol demands more than just stacking containers. Store it in a spot free from sunlight and heat. Keep it tightly sealed, far from anything even remotely flammable or reactive. I’ve seen leaks happen because someone decided to drag barrels over concrete, causing the weakest seam to split—a mess best avoided.
Treating dangerous chemicals like regular garbage or letting them slip down the drain has never ended well. Chemicals like Nonylphenol spread fast in water and soil, hurting fish, plants, and even your local drinking supply. Last year, a local river saw a spike in dead fish, and folks downstream worried about their tap water. Eventually, the story traced back to a small business dumping cleaner with Nonylphenol in it. The environmental officers spent weeks testing, cleaning, and reminding people what those hazard symbols mean.
Some factories try diluting their waste. That doesn’t make the threat go away; it pushes it somewhere else—sometimes underground, other times into air ducts. I remember a news clip from a few years back: a fire broke out at a scrapyard during a heat wave. Sealed barrels exploded, spreading chemicals all over the neighborhood. You couldn’t walk outside without smelling the release in the air. Cleanup cost the city thousands and the company even more.
If you’re working around Nonylphenol, don’t shortcut storage. Use containers that can handle a little rough treatment. Make sure labels stand out, and keep them current—no mystery liquids in unlabeled bottles. Ventilated and locked storage, with warning signs for workers and first responders, can stop an accident from turning into a disaster.
For disposal, professional help pays off. Companies who handle hazardous waste have the right tools and routes, sending leftovers to incineration or special treatment centers, not down a regular drain. Municipalities often offer hazardous waste collection events or guidance. I once helped at one of these drop-offs, and was struck by the number of folks dropping off unlabeled jugs, hoping someone else would deal with it. If people just try a little harder to read up and ask where to bring such stuff, trouble stays out of the environment.
Open records, clear instructions, and honest conversations with chemical suppliers or local environmental agencies turn the process from confusing to manageable. At the end of the day, people respect what they understand, and a community that expects clear chemical management sees far fewer headlines about spills, poisonings, or polluted water.
Nonylphenol has a knack for showing up in everyday things, especially in detergents, cleaners, and the world of plastics. Under the sink or behind factory doors, it’s easy to find. What many people don’t realize is this clear liquid doesn’t just mix in the water and disappear; it sticks around, clings to wildlife, and piles up in our rivers and streams. Scientists have kept a close eye on it, linking it to hormone disruptions in fish, dangers for workers, and even possible human health impacts.
Europe sounded the alarm early. Seeing fish populations shrink, with deformities and fewer females able to lay eggs, European regulators took real action. Around 2003, the European Union rolled out strict restrictions with its “Directive on Dangerous Substances.” Factories had to adapt, swapping out nonylphenol for less risky options. The rules grew tighter with REACH legislation, pressuring every industry to prove safety before anything lands on the market. If someone tries to use nonylphenol in cleaning fluids or textiles for the EU, inspectors and fines aren’t far behind.
The story takes a different route in the United States. Here, the Environmental Protection Agency placed nonylphenol on its chemicals of concern list. Rules set limits for how much can slip into surface waters, and several voluntary programs nudge industries away from older formulations loaded with this chemical. The US hasn’t banned nonylphenol outright, but nerves among shoppers and pressure from environmental groups have convinced big brands to move toward safer suds and plastics.
Japan made changes in the late 2000s, putting tough controls on the production and use of nonylphenol compounds. Several provinces in Canada banned its use in cleaning products and demanded new wastewater handling for factories. China moved slowly—concern picked up only after studies recorded nonylphenol traces in drinking water. Shanghai’s government forced local plants to cut back, setting a precedent for other big cities. These shifting lines worldwide make it clear that the mood is changing, even if there isn’t one global rulebook.
It’s obvious that some regions have made bold steps while others lag. Countries with less cash for environmental monitoring let a lot of nonylphenol slip through. The textile industries in Southeast Asia and parts of Africa still send this chemical down the drain without filters or oversight. This matters to anyone buying imported clothes or eating seafood from polluted rivers.
The best fixes don’t rely on one country or one ban. Companies can choose greener formulas, and shoppers can look for eco-friendly labels. Regulators should share test results and push companies—especially multinationals—toward cleaner practices even outside their home turf. Knowledge sharing makes a huge difference, from best wastewater treatment methods to smarter production chemistry. Real change comes when industries join hands with regulators and scientists, not just passing rules but finding practical, cost-effective replacements. Until these steps take hold everywhere, nonylphenol will keep making trouble, sneaking into water, fish, and maybe even dinner plates.
| Names | |
| Preferred IUPAC name | 4-nonylphenol |
| Other names |
phenoxynonane N-phenyl-nonanol isononylphenol p-nonylphenol 4-nonylphenol nonylphenol-4 n-nonylphenol p-nonylphenyl alcohol |
| Pronunciation | /ˌnɒn.ɪlˈfiː.nɒl/ |
| Identifiers | |
| CAS Number | 25154-52-3 |
| Beilstein Reference | 1361082 |
| ChEBI | CHEBI:28661 |
| ChEMBL | CHEMBL42978 |
| ChemSpider | 6577 |
| DrugBank | DB03809 |
| ECHA InfoCard | ECHA InfoCard: 100.002.166 |
| EC Number | 204-881-4 |
| Gmelin Reference | Gmelin Reference: **199416** |
| KEGG | C06538 |
| MeSH | D009732 |
| PubChem CID | 7258 |
| RTECS number | RB9620000 |
| UNII | 7T1F7V2K4Y |
| UN number | UN2810 |
| Properties | |
| Chemical formula | C15H24O |
| Molar mass | 220.35 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | characteristic phenolic |
| Density | 0.953 g/cm³ |
| Solubility in water | 1.6 mg/L |
| log P | 4.48 |
| Vapor pressure | 0.3 mm Hg (20 °C) |
| Acidity (pKa) | 10.7 |
| Basicity (pKb) | 7.84 |
| Magnetic susceptibility (χ) | -7.5×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.542 |
| Viscosity | 11-13 mPa.s (at 25°C) |
| Dipole moment | 4.63 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 528.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -340.65 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -6107.8 kJ/mol |
| Pharmacology | |
| ATC code | D08AX99 |
| Hazards | |
| Main hazards | Corrosive, harmful if swallowed, causes skin and eye irritation, toxic to aquatic life with long lasting effects |
| GHS labelling | GHS02, GHS05, GHS07, GHS08, GHS09 |
| Pictograms | GHS05,GHS07,GHS08,GHS09 |
| Signal word | Danger |
| Hazard statements | H302, H314, H410 |
| Precautionary statements | P260, P262, P273, P280, P301+P312, P305+P351+P338, P308+P313 |
| NFPA 704 (fire diamond) | 3-2-0 |
| Flash point | 170°C |
| Autoignition temperature | 330°C (626°F) |
| Explosive limits | Explosive limits: 1.4–9.8% |
| Lethal dose or concentration | LD50 (oral, rat): 1620 mg/kg |
| LD50 (median dose) | LD50 (median dose) of Nonylphenol: "580 mg/kg (oral, rat) |
| NIOSH | RN367S6000 |
| PEL (Permissible) | 5 mg/m3 |
| REL (Recommended) | 1 mg/m3 |
| IDLH (Immediate danger) | IDHL: 100 mg/m3 |
| Related compounds | |
| Related compounds |
Octylphenol Bisphenol A Alkylphenol ethoxylates Nonylphenol ethoxylate Phenol Cresol Tert-butylphenol |