Stories about everyday chemicals never catch much attention, but methyl acetate has an interesting past nestled in the evolution of solvents and industrial chemistry. Its roots stretch back over a century, cropping up as chemists searched for safer, less toxic alternatives to old-time favorites like benzene and carbon tetrachloride. Factories making lacquers and paints soon welcomed methyl acetate for its quicker evaporation and cleaner finish. I’ve seen old textbooks praising its odor as less harsh, a small mercy to workers before the days of effective masks. This shift from brute-force solvents to ones that offer efficiency with a smaller health bill shows progress in chemistry that’s both practical and personal; no one wants to end a shift dizzy from fumes.
Methyl acetate stands out for its flexibility in the world of solvents. Far from obscure, it plays a central role in coatings, adhesives, and nail polish removers. Industrial users value it because it mixes well with alcohols, ketones, and esters, broadening its reach. Manufacturers in the past often worried about sticky residues or slow drying, but methyl acetate dries fast and leaves surfaces crisp. Chemists — including myself in early lab jobs — often reach for it in synthetic work when tougher, more hazardous options just don’t fit the bill. Its pleasant, mild scent made it a favorite to test in workshops and classrooms before strict VOC rules forced tougher controls.
Looking at methy acetate’s key details, it comes clear why industries favor it. With a low boiling point around 57°C and a flash point close to -10°C, it evaporates rapidly, which matters in settings where time is money. It handles well under standard pressures and temperatures and doesn’t gum up equipment. Sparingly soluble in water but highly miscible with most organic liquids, it’s a go-to for jobs demanding speed and precision. Its molecular weight ticks in at just over 74 g/mol — not heavy, but not too light to require special handling. Having spent time measuring vapor concentrations, it’s easy to see its practicality for workers who want to clear a bench fast and move on to the next batch.
Grab a bottle of methyl acetate from a chemical supply room and its label reads like a chemistry cheat sheet. Purity usually sits above 99%, which means less trouble downstream in reactions and processing. Labels flag hazard classifications, UN number 1231, and highlight storage tips: cool, dry, away from sparks or open flames. Each drum needs health warnings about inhalation risks and instructions for quick clean-up after spills. Density information hovers near 0.93 g/cm³, with kinematic viscosity low enough to pour smoothly, which I’ve learned makes a big difference when pouring from unwieldy bulk drums during busy production runs.
Making methyl acetate usually starts with the reaction between acetic acid and methanol, in the presence of strong acid — sulfuric acid most of the time. This Fischer esterification is classic undergraduate lab fare. Adjusting temperature, tweaking the ratio, and using distillation, plants churn out high-purity product daily. Alternative routes using carbonylation flicker on the edges of research, but nothing beats the plain old acid-catalyzed method. I remember scaling up from test tubes to multi-liter batches, finding that side reactions rarely ruin yields if everything stays dry and clean. Cost matters, so the current methods hang on because they just work.
In reactions, methyl acetate mostly keeps to itself, but it won’t hesitate to hydrolyze back to acetic acid and methanol in the presence of water and acid or base. Over-exposure to strong bases can snap its bonds, while gentle processing lets it serve as a raw material for further esterifications and transesterification reactions. In specialty labs, tweaking its structure enables the formation of new esters, handy in fragrance or flavor development. I’ve watched plenty of students underestimate its volatility, only to end up with dried-out reaction mixes if left uncovered even a short spell.
Methyl acetate doesn’t hide behind many aliases, but you’ll see it show up as methyl ethanoate or acetic acid methyl ester in chemical catalogs. Trade names rarely stick, but safety data sheets sometimes favor the IUPAC version, causing a few headaches for those new to industrial supply. Walking through a storeroom, I spot these different names, but the clear, fruity smell always gives the substance away.
No one wants an accident on their hands, and methyl acetate demands caution. Its flammability keeps it on watch lists; vapors can ignite with a stray spark. Good ventilation and grounding for equipment come standard in labs and plants working with methyl acetate. Eyes and skin need protection because it causes irritation quickly — I learned this early, and keep a set of splash goggles nearby. Regulatory agencies, including OSHA and the European Chemicals Agency, set tight exposure limits to safeguard workers. Fire drills and spill response routines don’t get skipped in any facility that keeps the stuff on hand; even a small spill means springing into action, using foam or CO2 extinguishers and scrubbing down every surface.
Methyl acetate does heavy lifting in paints, coatings, cleaning agents, and adhesives. Its fast evaporation allows auto shops to finish jobs faster, while furniture factories appreciate how it levels finishes without clouding or streaking. In electronics, it cleans sensitive parts because it cuts grease but leaves negligible residue. Artists even leverage it to dissolve pigments in specialty inks. Speaking from experience, cleaning up after a workshop with methyl acetate takes less effort, and the results show it’s still ahead of slower, heavier solvents that tend to linger on surfaces and in the air.
Development teams now push to find ways to trim VOC emissions, and methyl acetate draws renewed focus. Its lower toxicity compared to older solvents makes it a candidate for greener production lines. Academic labs tinker with blends that swap out hazardous aromatics or chlorinated compounds, counting on methyl acetate’s reliability and ease of use. Researchers also test it as a base for new eco-friendly coatings or as a green solvent in pharmaceutical synthesis. My own collaborations with environmental teams have focused on emission measurement, balancing process performance with air quality targets. Anyone serious about reducing worker health claims and environmental burdens keeps methyl acetate on their short list.
Data from animal studies and industrial monitoring suggests methyl acetate holds lower acute toxicity compared to many of its solvent cousins. Repeated exposure can irritate the airways or eyes, but chronic long-term effects only turn up at exposure levels much higher than any modern workplace should allow. Agencies like the EPA and ECHA call for careful handling, but regulatory limits get set well below observed danger points. Toxicology teams continue tracking cases for signs of unexpected impacts as substitutes enter new markets. For now, replacing nastier solvents with methyl acetate looks like a health win in both factories and research settings.
With sustainability on everyone’s mind, methyl acetate looks poised for a busier future. Teams designing waterborne coatings watch it for unique blending options, while automotive and polymer industries explore its role in recycling and advanced material assembly. Combining low toxicity with strong solvency and quick flashing, it stands out among options with fewer drawbacks. Regulatory pressure on air emissions and workplace exposures could push more companies to switch over, especially as suppliers refine purification and develop variants with tailor-made evaporation rates. Anyone working in product development — as I continue to do — can see the edge methyl acetate brings over established but hazardous legacy solvents. In the push to clean up both air and supply chains, it offers choices that weren’t available to previous generations of chemists and factory workers.
Methyl acetate pops up in places people rarely notice. This colorless liquid helps make paint go on smooth and dry fast. Walk into a hardware store and scan through cans of lacquers or spray paint—chances are, there’s methyl acetate inside. Before working as a writer, I spent years on the shop floor of a woodworking business, where fast-drying paints and finishes made our jobs easier and let us finish projects on time. Without reliable solvents like methyl acetate, we'd still be waiting for cabinets to dry days after painting.
Step into a nail salon, the sharp scent in the air usually comes from nail polish removers. Methyl acetate helps dissolve the tough resins and dyes that give nail polish their color and shine. It works quickly and evaporates clean, so users aren't left with sticky residue or strong odors on their skin. At home, products like adhesives and even some cleaning sprays rely on the quick-acting nature of this solvent to break down sticky messes without much fuss.
Factories that manufacture plastics, artificial leather, and coatings count on methyl acetate to do heavy lifting behind the scenes. It acts as a carrier for chemicals, making it easier to apply thin layers of varnish or laminate without streaking or sagging. This detail makes industrial finishing lines much more efficient. In my own days handling inventory for a packaging company, I saw how production slowed to a crawl the minute we ran out of good solvents. Reliable performance means higher output—and that spells survival for any manufacturing business competing on tight deadlines.
The speedy evaporation that makes methyl acetate so useful has a flip side. The fumes, if not controlled, can make people dizzy or irritate eyes and lungs. Methyl acetate doesn't hang around for long in the environment. Compared to much harsher chemicals like toluene or benzene, this one breaks down fairly quickly. But if factory workers or hobbyists get reckless and use it indoors without fresh air, headaches or worse could follow. Seeing coworkers get sick from poor ventilation was a wakeup call for me—so I started paying attention to labels and opened a few extra windows.
Demand for “green” solvents grows louder every year, especially in cities where air quality matters. Some paint and adhesive companies have started blending methyl acetate with water-based solutions, so less of it drifts into the air. Designers also experiment with ingredients from pine trees and citrus fruits. These are not always as cheap or easy to use, but folks seem willing to pay a few extra bucks for peace of mind.
Regulations play a part. Places like California have rules that push manufacturers to rethink their ingredients. These rules drove innovation at the furniture plant I worked for—we started using safer cleaners and found they worked just fine. Changes like this, forced by both regulation and consumer preferences, help nudge the industry toward healthier workplaces.
Methyl acetate keeps showing up across industries because it works, and it keeps things moving. It would be hard to toss it altogether without losing efficiency. Still, more people want lighter smells and safer air, both at work and home. Smart handling, updated safety standards, and a steady search for better choices all help keep the balance between performance and peace of mind.
Methyl acetate sounds like the kind of thing you’d find only in a lab, guarded by serious people in white coats. In truth, millions come across this substance without even noticing. It's a colorless liquid, smells a bit like glue or nail polish remover, and pops up in paints, coatings, and cleaning agents. Anyone who's refinished a table with lacquer or stripped a surface might have gotten a whiff. Sometimes people see a chemical name and panic, but panic doesn't help anyone handle chemicals more safely.
Breathing in methyl acetate at home or on a jobsite can feel a lot like dealing with acetone or ethanol—nausea, dizziness, even headaches if you really inhale a lot. The safety data sheets aren’t exaggerating; at high concentrations, this stuff can burn your nose or make you lightheaded. Spill enough on your skin and you’ll feel dryness or maybe irritation, especially if you're not in the habit of gloves.
Methyl acetate evaporates fast. That's one upside. Outdoors or in a decent-sized, ventilated room, the risk drops because the fumes don't hang around. Problems grow where air gets trapped—in a closet, under a sink, or in a cramped workshop with the door closed. Sitting in that kind of space with a can of lacquer thinner turns into a bad idea pretty fast.
Forget the toxicity for a moment. This chemical is flammable enough to catch fire from a static spark or an overheated extension cord. If you’re spraying paint enriched with methyl acetate, you’ll want to clear away open flames and even go easy on power tools that spit sparks. Fire crews dread solvent fires because they spread quickly and often result in bigger property damage.
Methyl acetate isn’t hiding a deadly secret. The World Health Organization and environmental agencies have studied it—no cancer warnings, no ties to long-term nerve damage. The body breaks it down into acetic acid and methanol, both of which also show up in food and drink. Doesn’t mean it’s harmless: methanol can poison you in large doses, but getting a short snootful from cleaning windows doesn’t come close to that level.
For folks handling methyl acetate at work, the key isn’t fear; it’s habit. Crack a window, use an exhaust fan, take breaks outdoors—those steps don’t cost much but make a huge difference. Wear gloves if you’ve already got dry hands. Store the can far from heat or flame. It makes sense to keep children and pets clear, since they’re more likely to get exposed by accident.
In my own garage, I learned this lesson the sticky way. I left a lid off overnight and woke up to a sharp, glue-like smell—enough to drive people out of the house. That lingering odor isn’t just unpleasant. If I’d ignored it, I would have risked a headache or worse, all for the convenience of skipping a few extra turns of the cap.
Methyl acetate acts a lot like other household solvents—respect goes a long way. It rewards the careful user and punishes shortcuts. Fumes, fires, and spills are all manageable if you know what the chemical can do and gear up just a little. Understanding how it interacts with the body, air, and fire takes some mystery and a lot of fear out of the whole question.
A lot of folks might not recognize methyl acetate right away, but it shows up all over the place. This is the kind of solvent you find in glue, nail polish remover, and sometimes even in the lab fridge right next to other common chemicals. The boiling point—57°C or roughly 135°F—tells you how quickly this stuff can disappear into the air if left open. Leave the lid off a bottle, and pretty soon you'll just have to settle for the scent, not the liquid.
Lab work tells you pretty fast why knowing the boiling point matters. Back in college, knocking over a beaker and running into a clear patch of air that smelled fruity—almost sweet—reminded me just how quickly methyl acetate can become vapor. In industrial settings, that quick leap from liquid to gas comes with some baggage: it means you get fast drying rates for everything from paint to adhesives, but you also run smack into fire risk. Anything that evaporates that easily needs respectful handling.
Companies love solvents that get in, do their job, and then vanish without a trace on the finished product. The low boiling point lets methyl acetate shine here. Folk working in furniture finishing or automotive touch-up work like how fast it leaves surfaces ready for another coat. But the flip side enters when you realize how easy it is to end up with too much of it in the air, especially in smaller workshops or closed rooms. I’ve had days in a poorly ventilated garage where the air felt tinged with overused solvents, reminding me of the need to pay attention to local air quality standards.
This isn’t just about making sure your lacquer dries right. It’s also about protecting lungs, skin, and even the neighbors next door. Methyl acetate will irritate eyes and noses if you let too much hang around. Regulations set the tone for how much vapor you can have hanging in the air, and smart workplaces answer with good vent fans and by scheduling work to make sure no one sits surrounded by fumes for hours on end. Proper storage—no jerry-rigged lids and rusty cans—matters just as much, because this solvent evaporates quickly, especially if storage rooms get warm.
In lab work, folks often get casual with containers, only to find half the solvent gone after a weekend. My own early experiments sometimes turned into major cleaning jobs because I underestimated how volatile some chemicals could be. Over time, you learn to keep chemicals capped, label containers clearly, and set up good air movement—simple steps that mean less waste and less exposure.
Short trips around campus and in company shops make clear that good information goes a long way. Young techs ought to learn early on: don’t lean over open containers, don’t ignore that fruity smell, and always keep good air flow. Switching to less volatile alternatives won’t always fit every job, but options exist. Some workplaces rotate in other solvents or set up automated systems to minimize human contact and waste. Everyday habits—wearing gloves, keeping bottles sealed, setting up fans—make the difference between a safe project and a headache by midafternoon.
Methyl acetate looks unassuming, just another clear liquid in a chemistry lab or a list of industrial ingredients. It’s not famous like ethanol or controversial like acetone, yet somebody working in a paint shop, a scientist, or even a hobbyist mixing their own cleaning supplies has probably bumped into it. The question, “Is methyl acetate soluble in water?” sounds straightforward, but the answer has more practical weight than many people realize.
Lots of us ignore the names on bottles and cans as we go about our day, but many common products hold ingredients like methyl acetate. This stuff pops up in nail polish removers, perfumes, adhesives, and cleaning agents. I remember once watching my uncle strip old paint off a wooden chair, pausing to mix a cleaner and a solvent. The labels might as well have been written in another language for all the sense it made to me then. Later, in a college lab, figuring out whether something would mix with water became a crucial, sometimes messy lesson.
So does methyl acetate disappear into water or float on top? The answer is: it dissolves, but not without limits. According to chemical handbooks, methyl acetate reads out at around 25 grams per 100 milliliters at room temperature. It doesn’t blend like salt or sugar (which seem to vanish into water), yet it doesn’t sit on the surface like oil, either. What does this mean outside a lab? If you tip a little methyl acetate into a cup of water and stir, it will dissolve, but keep adding more and you’ll end up with layers.
Chemistry has a habit of slipping into regular life. If you’re working with solvents, hoping to clean up a brush, thin paint, or strip something off a surface, you want to know if water alone will carry away the leftover chemicals. If methyl acetate mixed freely in any amount, cleaning up would be a breeze—grab a bucket, soak everything, problem solved. Because it mixes only in certain ratios, you have to pay attention. The leftover solvent in brushes or rags won’t just rinse clean forever; at some point, the water won’t grab any more methyl acetate.
On top of everyday cleanup, industries depend on this kind of chemistry to avoid environmental hassles. Factories handling methyl acetate need to treat even their wash water with care, since water alone won’t always carry away every drop. Wastewater limits from regulators mean you can’t release the stuff into drains just because you used water to dilute it. Understanding solubility can keep fines and pollution headaches at bay—and that’s a lesson I’ve seen learned the hard way on more than one shop floor.
There are good ways to work with this middle-of-the-road solubility. Mixing methyl acetate with water helps reduce danger and provides a route for safer handling. Adding just the right amount of water can turn a sticky mess into something washable. Beyond a certain point, though, adding more water doesn’t fix the problem—you need to use absorbents, try different chemicals, or use special disposal techniques.
Straight answers about things like solubility save time, prevent mistakes, and keep workers safer. Knowing how far you can push a mixture before it stops working isn’t just about chemistry, it’s about making work and cleanups simpler and safer.
Methyl acetate is a clear liquid with a fruity smell, perfect for pulling nasty residues out of machinery or mixing into coatings. That familiar odor signals volatility—this stuff wants to be airborne. Plenty of folks underestimate that. Back when I worked in a warehouse filled with drums of chemicals, the new guys would joke about the rotten apples scent. No one likes playing chemistry teacher, but it’s easy to forget a pleasant smell doesn’t mean harmless.
Storing methyl acetate without strict rules is a recipe for trouble. Flammable is putting it mildly. A single leaking drum near a stray spark could level a shop. Insurance adjusters have seen this before, and the aftermath is never pretty. OSHA and the fire marshal don’t shrug off mishaps here. It’s not about avoiding fines—people’s lives are tangled up in careless shortcuts.
I remember one summer, temperatures soared, and our storage shed turned into a sauna. We had barrels of methyl acetate sitting too close to a window. I learned real quick—heat makes those vapors aggressive. Soon the fire alarm tripped; we aired out the place in a panic, moving everything to a shady corner. Shade and airflow kept the fumes from building up, and we ditched anything that started looking rusty or dented.
Methyl acetate belongs in tightly sealed metal drums or approved polyethylene containers. Cracked seals or makeshift lids aren’t just wrong—they’re invitations for fumes to seep out, especially in muggy climates. I’ve seen more than one rookie try to reuse old paint buckets. It isn’t worth the risk. Dedicated containers, clearly labeled and never stacked more than two high, keep leaks from turning small mistakes into emergencies.
Put these drums on spill pallets with raised edges. You never notice your floor slopes until something starts dripping. A proper pallet keeps any mess from spreading. In my old place, there was a habit of brushing spills aside, thinking nothing of it. Cleanup powders take care of small splashes, but only if workers know where to find them and don’t treat cleanup like a chore for the next shift.
Avoiding closed spaces is non-negotiable. Any leftover fumes can turn a simple flick of a light switch into an explosion. Fans on the wall or open vents at the roofline keep air moving and reduce build-up. Everyone thinks it won’t happen to them, but fires move fast. I’ve seen storerooms with half-melted light fixtures after someone ignored a faulty wire.
Every container should spell out its content. Wishful thinking and faded labels lead to mistakes and confusion. Safety data sheets pinned by the door might gather dust, but reading through them once saves plenty of headaches. Eye wash stations and emergency showers don’t belong in a back closet; they get top billing near storage areas.
Training beats guessing. It doesn’t take hours out of the month—a few minutes before each shift covers most of what people forget or never learned. The risks aren’t theoretical. Methyl acetate can go from manageable to deadly in a worst-case scenario, but with some care, those disasters remain stories and never become headlines.
| Names | |
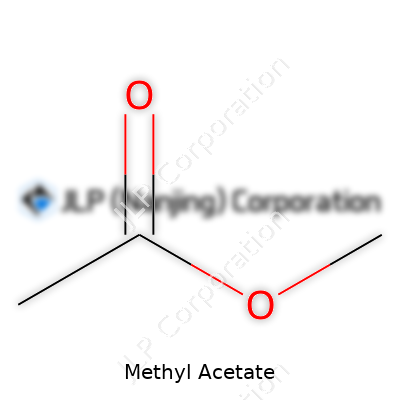
| Preferred IUPAC name | methyl ethanoate |
| Other names |
Acetic acid methyl ester Methyl ethanoate |
| Pronunciation | /ˈmɛθɪl ˈæsɪteɪt/ |
| Identifiers | |
| CAS Number | 79-20-9 |
| 3D model (JSmol) | `CCC(=O)OC` |
| Beilstein Reference | Beilstein 1731153 |
| ChEBI | CHEBI:7929 |
| ChEMBL | CHEMBL14036 |
| ChemSpider | 7867 |
| DrugBank | DB11254 |
| ECHA InfoCard | 03bfa5b0-9a70-47c4-8f43-d780a7faf787 |
| EC Number | 123-539-8 |
| Gmelin Reference | 7958 |
| KEGG | C01801 |
| MeSH | D008736 |
| PubChem CID | 7907 |
| RTECS number | AI0175000 |
| UNII | 86C2EA4C3Y |
| UN number | UN1231 |
| Properties | |
| Chemical formula | C3H6O2 |
| Molar mass | 74.08 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | fruity |
| Density | 0.93 g/cm³ |
| Solubility in water | 25.0 g/100 mL (20 °C) |
| log P | 0.18 |
| Vapor pressure | 181 mmHg (20°C) |
| Acidity (pKa) | pKa ≈ 25 |
| Basicity (pKb) | The basicity (pKb) of methyl acetate is **-**. |
| Magnetic susceptibility (χ) | -43.5e-6 cm³/mol |
| Refractive index (nD) | 1.361 |
| Viscosity | 0.41 mPa·s (at 25°C) |
| Dipole moment | 1.72 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | S⦵298 = 283.5 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -413.3 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -879.1 kJ/mol |
| Pharmacology | |
| ATC code | V07AB |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02, GHS07 |
| Signal word | Warning |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P261, P264, P271, P280, P301+P312, P303+P361+P353, P304+P340, P305+P351+P338, P312, P337+P313, P370+P378, P403+P233, P403+P235, P405, P501 |
| NFPA 704 (fire diamond) | 1-3-2-– |
| Flash point | -10 °C |
| Autoignition temperature | 454 °C (849 °F) |
| Explosive limits | 3.1% - 16% |
| Lethal dose or concentration | LD50 oral rat 6,482 mg/kg |
| LD50 (median dose) | LD50 (median dose): 5,000 mg/kg (oral, rat) |
| NIOSH | AI9825000 |
| PEL (Permissible) | 200 ppm |
| REL (Recommended) | 200 ppm |
| IDLH (Immediate danger) | 3100 ppm |
| Related compounds | |
| Related compounds |
Acetic acid Methanol Ethyl acetate Methyl formate Acetone |