Ethyl acetate traces its roots back to the wave of chemical experimentation in the 18th and 19th centuries, when the urge to understand the science behind substances drove early chemists to tinker with acids and alcohols in makeshift labs. I’ve still got vivid memories from university labs where the legacy of early experiments hung in the air—those scientists used apparently simple recipes, pouring acetic acid over ethanol, then carefully separating the layers to isolate a volatile, sweet-smelling product. Over time, synthetic routes changed, but the core chemistry and pursuit of better purity and yield never left the agenda. Industrial production ramped up after the advent of large-scale distillation and more precise temperature control—two things the mid-1800s crowd could only have dreamed about. Watching this compound move from hobbyist curiosity to factory staple tells a lot about the way basic discoveries scale into crucial industrial tools.
You see ethyl acetate in food processing, pharmaceuticals, inks, and even nail polish removers. Its fruity aroma carries into the flavoring world and helps shape the scent in perfumes just as much as it dissolves resins and varnishes elsewhere. Every time I’ve opened a can of paint thinner or sniffed an artificial fruit flavor, I’ve caught a hint of ethyl acetate at work. Companies look for it because this single substance bridges lab, factory, and everyday products without becoming too hazardous or hard to handle. There are fancier alternatives out there, but few share the same balance of effectiveness, cost, and manageable safety profile.
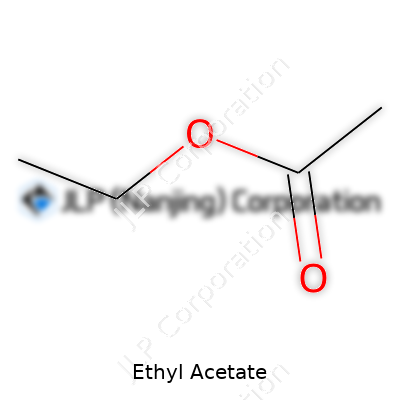
Ethyl acetate presents itself as a colorless liquid that evaporates with enthusiasm, leaving behind its telltale smell. Its boiling point sits conveniently at about 77°C, making it useful in processes that rely on easy evaporation or separation. Immiscible with water at high concentrations, but still able to dissolve a wide array of organics, it sometimes acts as a go-between for mixtures that don’t usually play well together. Chemically speaking, its structure—CH3COOCH2CH3—shows how simple tweaks on carbon backbones unlock a diverse pool of uses. I’ve learned the hard way that its flammability means treating it with more caution than the average non-polar solvent. Spills vaporize quickly, raising fumes and fire risk, so lab ventilation and careful storage always matter.
Any drum of ethyl acetate comes plastered with labels noting purity (typically above 99.5% for most technical grades), density (around 0.902 g/cm3), and hazard status. MSDS sheets from manufacturers spell out limits for exposure, recommend gloves and goggles, and list compatibility warnings, especially regarding acids or oxidizers. I once saw a batch rejected over trace levels of residual acid, which underscores the strict specifications for any product destined for food or pharmaceutical use. Supply chains hinge on reliable quality and documentation, not just a barcode and a safety symbol.
Most plants churn out ethyl acetate by quietly combining ethanol and acetic acid under sulfuric acid catalysis in a process called Fischer esterification. The alternative—transesterification between ethyl alcohol and acetic anhydride—finds use if higher purity is needed. Tiny changes, like temperature, water removal, or reflux timing, boost yield and save effort downstream. Home experimenters once produced small batches with a flask and condenser, but factories today use sophisticated reactors that recover and reuse heat and solvent, making production far less wasteful than the old days. Each tweak to the process means less waste, more profit, and fewer purity headaches.
In my own experience, ethyl acetate doesn’t shy from transformation. Under alkaline conditions, it undergoes hydrolysis, breaking down to ethanol and sodium acetate. Strong acids turn it back into its parent alcohol and acid in a reversal of its initial formation. In organic synthesis labs, I’ve watched it step in as a starting point for more complex molecules by reacting with Grignard reagents or halides. Chemists tinker with it to yield esters with slightly different chain lengths, showing its flexibility as a building block in bigger schemes. Changing one or two elements can produce solvents with altered volatility or reactivity, highlighting ethyl acetate’s place as more than just a finished solvent—it doubles as a starting material for inventive routes.
Flip through catalogs and you’ll see ethyl acetate called ethyl ethanoate, acetic acid ethyl ester, or even “EA” in shorthand lab speak. Commercial chemical vendors might badge it as “ethyl ester of acetic acid” or tuck it under house brand names, but no matter the label, the bottle always brings the same sweet, solvent punch to the party. The proliferation of names often stemmed from prior naming conventions, regional habits, or regulations. Reading some older research papers or industrial manuals really requires a mental dictionary just to cross-verify that each synonym actually means what you expect.
Ethyl acetate brings risks like flammability, potential irritation to skin or eyes, and inhalation hazards. Safe working spaces call for solid ventilation, strict no-smoking rules, and spill kits within easy reach. I’ve worked with solvents where one wrong move leads to significant vapor buildup and headaches for everyone in the vicinity. Factory operators and lab workers watch for leaks and keep fire extinguishers close. There’s also routine air monitoring because exposure above 400 ppm (in occupational settings) raises health concerns. Good training and clear signage make the difference between a routine day and a trip to the emergency room. Waste solvent collection tanks help prevent environmental releases, especially since uncontrolled draining damages waterways and wildlife.
Ethyl acetate’s uses stretch way further than one might expect. The printing industry relies heavily on its solvency to mix and fix inks, while pharmaceutical manufacturing uses it to extract active molecules and create targeted formulations. Food processing depends on strict quality checks since the same compound flavoring artificial bananas or pineapples in candy can pose harm if contaminated. In lab syntheses, it provides a go-to medium for extractions, washes, and purifications. Painters, varnishers, and nail technicians see its effects daily—quick-drying finishes, glossy coatings, or residue-free nail polish. Environmental testing even uses it to separate organic pollutants from water samples. Every field puts its own spin on what this versatile compound can do, but the thread tying them all together lies in its blend of low cost, easy removal, and broad compatibility with other chemicals.
Over the past decade, research shifted toward greener production. Collaborative work between universities and industry now focuses on bio-based routes, using fermentation to generate raw materials that leave a lighter footprint on resources and emissions. Enzyme catalysis promises processes that demand less energy by operating at milder conditions. Development teams test new stabilizers to extend shelf life or reduce emissions during storage and transit. Having participated in a student project comparing traditional and bio routes, I saw firsthand how cost, scale, and regulatory hoops present as much a hurdle as pure chemistry. Labs working on molecular modifications often look for ways to tune properties like evaporation rate for specialty coatings or adjust toxicity profiles for safer workplace handling.
Toxicological data on ethyl acetate, collected over decades, make a strong case for limited risks at low exposures but highlight dangers with higher doses or chronic exposure. Short-term contact rarely goes past mild irritation or drowsiness from breathing vapors; trouble comes from sustained headaches, nervous system effects, or organ strain with repeated contact. Long-term studies explore potential carcinogenicity and neurotoxicity but tend to show lower risk than more notorious solvents like benzene. Researchers follow evolving occupational exposure limits and develop more sensitive biomonitoring techniques. The experience of handling accidental overexposure—burning eyes and coughing—drives home the importance of regular environmental monitoring and personal protective equipment. Ongoing research aims to close knowledge gaps for low-level chronic exposures, especially in workplaces where other pollutants muddy the toxicology picture.
Looking ahead, the future of ethyl acetate likely revolves around sustainability and smarter process control. The chemical industry faces stiff pressure to reduce waste, cut dependence on fossil sources, and lower emissions. Switching feedstocks to renewable biomass keeps gathering momentum, especially as governments push for circular economy strategies and restrict emissions more fiercely. Research groups work on catalytic systems that deliver higher selectivity and lower byproduct formation, which shrinks costs and environmental liabilities. Quality assurance and real-time analytics develop in parallel, letting producers tweak processes on the fly for tighter tolerances. In consumer markets, there is interest in making products with lower residual solvent levels, and in manufacturing, automation and digital monitoring step in to catch small equipment or process faults before they cause big safety incidents. The global spread of new regulations will push both manufacturers and end users to close the loop on solvent use, recycling and recovering material wherever possible. In all cases, innovation keeps centering around making an old industrial workhorse like ethyl acetate cleaner, safer, and smarter, ensuring its place in modern life.
People might not recognize it by name, but plenty have caught that familiar whiff—fruity, a bit like pear drops, sometimes like nail polish remover. Ethyl acetate pops up in everyday life far more often than most would guess. It works quietly behind the scenes, playing a major role in flavors, fragrances, and even in something as simple as removing a stubborn sticker from a glass jar.
Those who bake or love candy have already met ethyl acetate. The food industry uses it to help create artificial fruit flavors. If you’ve sipped on a soda or chewed fruity gum, chances are this compound helped make that happen. It doesn’t leave any strange aftertaste or residue—just does its job and disappears.
Now, turn to the world of beauty and personal care. Open any salon, and the sharp smell drifting from a closed bottle of nail polish remover brings ethyl acetate front and center. It helps dissolve tough lacquer with little fuss. It’s less harsh than some other solvents and evaporates fast, so fingertips dry quickly instead of being left sticky.
In manufacturing, ethyl acetate helps dissolve glues, paints, and inks. I’ve seen it work wonders in art supply shops, cleaning up stubborn paint from brushes with minimal effort or thinning out oil-based paints without distorting their color. Commercial printers rely on it, too. It handles those demanding inks without breaking a sweat, making sure images come out crisp and colorful.
Turn to decaffeination, and you’ll stumble onto a surprising use. Coffee beans often sit in baths of solvents during the process. Ethyl acetate, sometimes nicknamed the “natural” process solvent since fruits produce small amounts of it, helps pull out caffeine without injecting a heavy chemical taste. Many health-conscious customers prefer beans prepped this way, as the residue left behind after processing sinks well below any risky levels.
It’s not all smooth sailing. Working with ethyl acetate carries its fair share of headaches—sometimes literally. Too much exposure leads to dizziness or sore throats, especially inside poorly ventilated spaces like small workshops or garages. I always crack a window and grab a good mask whenever opening a new can. Safety goggles do more than just keep dust out—they stop accidental splashes, which sting worse than lemon juice in a paper cut.
The world doesn’t stand still. Many industries look for greener options. Some switch over to water-based solvents, reducing their need for ethyl acetate or reserving it for the jobs nothing else can touch. Recycling systems in paint factories now catch and reuse this solvent, which saves money and keeps hazardous waste down. The food and fragrance industries, meanwhile, have strict guidelines about how much can stay behind in a finished product—safety remains the focus.
Ethyl acetate isn’t some fleeting trend—it’s built into the rhythm of daily living, humming away in the background. From making morning coffee less jittery, to keeping nails in top form, or thinning a stubborn glob of craft paint, it quietly shows up. It pays to understand what moves through our homes and bodies, and to handle this sweet-smelling helper with both respect and a little healthy caution.
Walk into a nail salon. That sweet, fruity smell hanging in the air? Sometimes, it's ethyl acetate. Open a bottle of some nail polish remover or glue, and there it is again. Companies put ethyl acetate in all sorts of household goods—varnishes, paints, food flavorings—even decaf coffee relies on it for processing.
Questions about safety often pop up because this chemical lands on ingredient lists everywhere. If regular folks use it at home, and workers deal with it in factories, everyone deserves straight answers. I often think about the times I cleaned paintbrushes as a kid, noticing how quickly that sharp odor made me dizzy. That stuff gets into your lungs fast. Something with such a strong smell can’t ever be ignored.
Breathing in those vapors makes your nose run or eyes sting. If you use it in a tight space, headaches aren’t rare. At higher doses, it can even knock you out or cause long-term problems. Spilling a bit on your skin leaves you with dryness and irritation. The liquid evaporates fast, which means it goes straight into the air, ready for anyone nearby to breathe.
Fire risk shows up wherever ethyl acetate sits open too long. This stuff catches fire at a low temperature—lighting a cigarette or hitting a spark nearby could turn careless handling into a real emergency. People might not think much when opening a jar in the house, but accidents can turn ugly fast if someone isn’t paying attention.
Ethyl acetate shows up in food and drinks. The amounts are tiny there, though, far below what happens in a workplace or a badly ventilated art studio. Some folks look up the name and panic, thinking it’s deadly in all forms. Most data, including studies from the Environmental Protection Agency and the European Chemicals Agency, agree that low-level skin or mouth exposure hardly causes major harm. The real danger shows up with high vapor levels and repeated contact.
Factory workers and salon technicians live with ethyl acetate all day. They can’t just crack a window and hope for the best. Protective gloves and good fans help, but those things break down or get ignored. Training falls short, especially when new employees come in and rush their tasks. It’s too easy to reach for speed rather than safety. At home, hobbyists rarely crack open safety data sheets. Little details, like keeping the workspace away from heaters or stoves, slip through the cracks.
A solution starts with simple habits, not fancy gear. Reading the label goes a long way. Open windows wide. Toss cheap latex gloves for nitrile ones if you’re painting or gluing. Never mix or use near flames—even pilot lights under the stove matter. You wouldn’t run a car in a closed garage, so don’t use chemicals in a tiny closet.
After years in creative spaces, from art studios to labs, I’ve seen that most mishaps happen when people forget what they’re handling out of routine. Taking five extra seconds to wipe up spills or switch gloves beats a day at the ER. When in doubt, call poison control or check credible sources. Ethyl acetate fits into life without drama, but only if people respect what’s in the bottle.
Ethyl acetate sits on plenty of chemical shelves, popping up in everything from nail polish removers to inks. In labs, folks know its banana-like smell, and folks on the factory floor recognize just how flammable it can get. Tossing a drum of this stuff in any old back room or cabinet simply invites trouble. Even in small leaks, vapors spread fast through the air, and one careless spark could turn a routine day into an emergency.
A smart approach starts with the right container. Drums or tanks using stainless steel or carbon steel take the daily knocks and seal tight. Lids must stay secure since this liquid evaporates quickly, and whatever comes out floats in invisible clouds — not exactly pleasant around ignition sources. I've worked in places where one loose lid led to stinging eyes all day, and folks had to air out the whole area.
Never treat this as household cleaner — don't store supersized drums in warm spots. Temperature creeps up, pressure in sealed containers builds, and those drips around valves or seams become real dangers. I once saw plastic containers slump after a summer weekend in a warehouse. So the storage space must keep it cool and shaded. Think well-ventilated, too, because vapor build-up doesn’t just linger – it crawls along floors since it’s heavier than air.
Stacking drums in a crowded space sets up a domino effect if one falls or leaks. Spacing matters. Store it away from heat, direct sunlight, and out of accident-prone travel paths. Don't squeeze containers tight against each other. Shelves or pallets that handle unexpected leaks keep the rest of the stock safe.
No matter the technical setup, success depends on people. Every worker needs to know not just the warning label, but what to do if there's a spill — how to find the nearest eye wash or emergency shower, who to call, and when to evacuate. Protective gloves and goggles stop skin and eyes from burning, which everyone who’s ever splashed it on their skin knows isn’t just a minor irritation.
Straightforward signage helps, too. Keeping big, clear labels and instructions close at hand means even new folks can react fast if things go sideways. There’s always the temptation to cut corners because someone’s in a hurry or thinks they've done it a hundred times, but real disasters often start small.
Agencies like OSHA, along with local fire codes, lay out exact storage distances from ignition sources, and cap the amounts you can stack in any one place. These rules didn’t appear out of nowhere — they came from real fires, real accidents, and lessons paid for in blood and lost time. Following them isn’t just about avoiding a fine. It’s about people heading home safe at the end of the day.
Waste needs careful handling too. Pouring leftover ethyl acetate down the drain isn’t just bad for waterways — it can spark up right in the pipes. Proper disposal setups, labeled drums, and timely removal keep the whole area safer.
Plenty of chemical incidents boil down to storage errors. Choosing a safe spot, using the right containers, watching the temperature and airflow, and making sure guests and workers know the risks helps keep the workplace safe and neighborhoods untroubled. The cost of a little extra care is nothing compared to the cost of a fire or an injury. That’s a lesson nobody should have to re-learn the hard way.
Ethyl acetate lands in so many places—paint thinners, nail polish removers, lab work. The moment you open a fresh drum, maybe in a workshop or a school lab, that sweet, fruity smell drifts up and lets you know something volatile is at hand. This stuff isn’t just a workhorse solvent; it’s a liquid that likes to go places fast, evaporating before your eyes if left uncovered.
Nobody wants to waste money or play dice with safety. Retailers usually stamp ethyl acetate bottles with a two-year shelf life, if not a bit more. From my own days working in a busy print shop, that timeline felt realistic. After about two years, bottles that weren’t tightly capped seemed to show something off: slower evaporation, strange odors, sometimes even yellowish tinges inside what should be a completely clear liquid.
Oxygen and humidity play the villains here. Left alone on a sun-baked shelf, a half-empty container does what any volatile solvent does: it breathes in little bits of moisture and oxygen each time you crack it open. That makes it slowly lose the crisp chemical punch you’re looking for. One study by the American Chemistry Council pointed to increased hydrolysis after just eighteen months in poor storage. That means a batch can quietly spoil long before the “expiration” date.
Nobody needs fancy lab equipment to spot a tired batch. If you pour some out and see it’s gone cloudy or if the sharp solvent smell has dulled, it’s time to move on. I’ve watched chemists reach for brand-new bottles rather than risk it; they trust their nose more than a dusty old label.
Keeping ethyl acetate in its prime sounds simple, but it’s where many slip up. You want it sealed, in a cool spot, out of sunlight. Metal drums with proper linings outlast plain plastic jugs. My old shop stored our solvents in metal safety cabinets, and I can almost guarantee those bottles lasted longer compared to others left right next to open windows. Never store it near acids or caustics—those cheaper, generic containers react with fumes, and I’ve seen barrels lost to cross-contamination.
Small operations sometimes buy more than they can use within a year, thinking it saves costs. Every expired jug argues otherwise. Smarter practice splits orders—keep one unopened, use up the current one fully, and cycle through stock.
Once you realize you’ve got a stale batch, don’t pour it down the sink. Local rules often treat it as hazardous waste. At the community paint collection point in my town, expired ethyl acetate joins everything else destined for safe handling. The environment doesn’t need extra volatile organics in the water system. Waste handlers will thank you for keeping things as labeled and capped as possible.
Solvents don’t last forever. Respecting their limits doesn’t just make for a safer garage or better lab results—it protects your wallet. It’s a lesson that keeps showing up across industry, research, and weekend projects alike.
Ethyl acetate often shows up in labs, factories, and classrooms. Plenty of folks buy it thinking, “pure is pure” as long as the bottle’s sealed and the liquid’s clear. That kind of thinking just opens the door for headaches down the line. I’ve stood over messy reactions in the lab enough times to know a little water or an unexpected impurity can derail an entire experiment. So whenever anybody asks about the purity of ethyl acetate, my answer is: know more than the label shows.
A bottle marked “analytical grade” usually goes above 99.5% pure. Chemistry teachers, pharma techs, and research scientists lean toward this because they remember the sting of failed tests thanks to the other 0.5%. The rest can be moisture, ethanol, or anything else left from the manufacturing process. Lower grade versions, sometimes called “technical” or “industrial,” hover closer to 99% or dip a bit beneath. That difference can mean the world in certain jobs where even a drop of impurity triggers a cascade of problems.
Last year, a small local paint shop learned the hard way. They mixed batches using cheaper, less pure ethyl acetate. Blisters formed in their lacquer, complaints rolled in, and their reputation took a hit. They hadn’t realized a few percent of extra stuff in the solvent could upset the whole operation. Cheap may look good upfront but, as they learned, hidden costs wait around the corner.
The numbers printed on a certificate shouldn’t get all of our trust without healthy skepticism. Storage conditions matter. Open the bottle too many times in a humid storeroom and water sneaks in. Suppliers sometimes cut corners or slip in a mix that passes basic tests but carries trace elements that only show up during sensitive runs. That’s why I ask for a recent quality analysis, not just the grade. If they can’t provide batch numbers and fresh data, I look elsewhere.
Consistent quality depends a lot on the supply chain. Producers who skip regular inspection do nobody any favors. I trust suppliers who trace every batch, test for common impurities like water and alcohol, and keep records open. Temperature-stable storage and sealed, tamper-proof packaging also make a difference. Some go a step further, inviting customers to inspect the process. That level of transparency doesn’t just help me sleep better—it means projects finish without surprises.
Anyone using ethyl acetate for serious work should kick the tires before committing. Ask for specifics on purity. Demand detailed certificates with each order. Set some solvent aside for your own quick check with a detector or titration kit. If problems pop up, trace them back: Was the storage leaky? Did moisture seep in? These steps take a little time but save plenty of money and stress later.
Ethyl acetate’s purity isn’t just a number on a sheet. It determines whether a batch passes inspection, a product earns trust, or costly surprises erupt. Real work means asking questions that go beyond the basics, holding suppliers to high standards, and paying attention to every part of the process from warehouse to workbench. Those who do, usually discover peace of mind and better results.
| Names | |
| Preferred IUPAC name | Ethyl ethanoate |
| Other names |
Acetic acid ethyl ester Ethyl ethanoate Ethylester kyseliny octové Essigsaeureaethylester Acetico etile Éster etílico del ácido acético Aethylacetat |
| Pronunciation | /ˈiːθɪl ˈæsɪteɪt/ |
| Identifiers | |
| CAS Number | 141-78-6 |
| 3D model (JSmol) | `Ethyl Acetate 3D model (JSmol) string`: ``` CCOC(=O)C ``` |
| Beilstein Reference | 635995 |
| ChEBI | CHEBI:27750 |
| ChEMBL | CHEMBL277481 |
| ChemSpider | 6912 |
| DrugBank | DB03157 |
| ECHA InfoCard | 03bfa4ee-6804-457d-b03d-3ec6e9e6a50e |
| EC Number | 205-500-4 |
| Gmelin Reference | Gm. 4028 |
| KEGG | C01015 |
| MeSH | D005001 |
| PubChem CID | 8857 |
| RTECS number | AH3325000 |
| UNII | NFY39M844R |
| UN number | 1173 |
| CompTox Dashboard (EPA) | DTXSID2020536 |
| Properties | |
| Chemical formula | C4H8O2 |
| Molar mass | 88.11 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | fruity |
| Density | 0.897 g/cm³ |
| Solubility in water | 8.3 g/100 mL (20 °C) |
| log P | 0.73 |
| Vapor pressure | 74 mmHg (20°C) |
| Acidity (pKa) | pKa ≈ 25 |
| Basicity (pKb) | 11.5 |
| Magnetic susceptibility (χ) | −40.6×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.370 |
| Viscosity | 0.45 mPa·s |
| Dipole moment | 1.78 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 160.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -483.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2219 kJ·mol⁻¹ |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02, GHS07 |
| Signal word | Warning |
| Hazard statements | H225, H319, H336 |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P261, P264, P271, P280, P301+P312, P304+P340, P305+P351+P338, P312, P337+P313, P332+P313, P362+P364, P370+P378, P403+P235, P501 |
| NFPA 704 (fire diamond) | 2-3-0 |
| Flash point | -4°C (25°F) |
| Autoignition temperature | 427 °C |
| Explosive limits | 2.1% - 11.5% |
| Lethal dose or concentration | LD50 oral rat 5,620 mg/kg |
| LD50 (median dose) | LD50 (median dose): 5,620 mg/kg (rat, oral) |
| NIOSH | KK8225000 |
| PEL (Permissible) | 400 ppm (TWA) |
| REL (Recommended) | 400 ppm |
| IDLH (Immediate danger) | 2000 ppm |
| Related compounds | |
| Related compounds |
Acetic acid Ethyl alcohol (Ethanol) Methyl acetate Butyl acetate Isopropyl acetate Propyl acetate |