Acrylamide-sodium acrylate didn’t spring into the world fully formed. Throughout the 20th century, chemists turned their sights from natural polymers to synthetic cousins, chasing better ways to control water, gels, and tough materials. Teachers ran basic polymerization reactions in high school chemistry labs, but the real breakthroughs happened in industrial research. In the 1970s, with the need for disposable diapers skyrocketing and agriculture searching for better water retention, new types of acrylate-based copolymers started hitting the scene. The rise of acrylamide-sodium acrylate reflects the deep appetite for practical chemistry, not just in textbooks, but in farms, hospitals, and manufacturing floors. Today’s tech for producing these copolymers didn’t appear overnight; it’s the result of decades of research into monomer behavior, safe manufacturing, and reliable performance.
Ask anyone who’s handled superabsorbents—acrylamide-sodium acrylate gets brought up for its ability to soak up water far beyond its own weight. Think powder that swells to a squishy gel. This makes it a core part of baby diapers, wound care pads, leak-proof packaging, and even gardening products. While it sounds niche, the amount of acrylate-based copolymer packed into daily life would surprise most people. To a factory manager, it comes as bags of powder or granules, ready to be mixed and molded. Its use speaks to a real need—keep liquids where you want them and not where you don’t.
Nobody picks up acrylamide-sodium acrylate for its looks—white to off-white powder, little to no odor, pours like sugar but turns into a soft jelly with a splash of water. The polymer backbone owes its strength to those double bonds that linked up during polymerization. Technicians spot its ionic nature right away—sodium acrylate brings negative charges, so it grabs water molecules, hanging onto them tightly. The copolymer stands up to salt and moderate heat, but break it down enough and acrylamide’s lingering risk starts to show: its monomer can cause trouble. So, people stay alert to monomer content, knowing repeated exposure isn’t just a regulatory concern but a real health risk.
Anyone reading a technical data sheet for acrylamide-sodium acrylate meets a wall of numbers: polymer molecular weight, water absorbency (up to several hundred times its own mass for the best grades), residual monomer content, particle size, cation exchange capacity. These aren’t window dressing. Water absorbency sets performance in hygiene products, while residual acrylamide content will decide whether a product heads to a baby store or needs strict workplace control. On bags and barrels, labels reflect real hazards—not just batch number and composition, but warnings for skin contact, dust inhalation, proper storage, and what to do if things spill. Regulatory certifications from places like REACH, the EPA, or China’s Ministry of Environment don’t show up just for show; lacking them means no entry into most markets.
Large-scale preparation of acrylamide-sodium acrylate isn’t the realm of lone scientists with beakers; this plays out in heavy-duty reactors under strict temperature control. The process starts with dissolving acrylamide and acrylic acid or sodium acrylate in water, then adding a crosslinker—often N,N'-methylenebisacrylamide—for that network structure. Free-radical initiators like ammonium persulfate trigger chain reactions faster than most people can imagine, locking monomers together and crosslinking until there’s a chunky, swollen gel. Afterward, crews break it up and dry it to produce those classic granules or powders. The reaction spits out heat and requires constant monitoring; a runaway polymerization can mean real disaster, with spills, burns, and lost batches.
Put acrylamide and sodium acrylate together under the right conditions, and crosslinks start forming all over the place, making the final gel strong and tough. Companies often tweak the base polymer, grafting side groups for new features—extra charge density, better resistance to ion interference, customized swelling. In some cases, the polymer undergoes hydrolysis to further fine-tune absorption or to reduce cost by starting from acrylamide and converting it later. These modifications don’t happen only to keep patents alive—they directly impact how well the material works in tough industrial settings, where every extra minute of water retention can mean more crops or fewer leaks.
Acrylamide-sodium acrylate doesn’t just go by the book name. People in labs talk about it as an acrylate copolymer, sodium polyacrylate blend, or superabsorbent polymer (SAP). Trademarks and brands—like Favor, Sanwet, SumikaGel—show up on packaging. Some older documents call it hydrogel or absorbent gel, especially in medical research. These aliases matter because regulations, contracts, and import paperwork can use different terms and still mean the same stuff. Miss those details, and shipments vanish into customs limbo.
Nobody working in a factory full of acrylamide-sodium acrylate wants to gamble with safety. Acrylamide monomer itself is a known neurotoxin and probable carcinogen, so tight controls keep its presence below regulated thresholds, usually below 0.05%. Good ventilation, gloves, goggles, and package handling routines make spills rare. In larger operations, continuous monitoring, proper lockout/tagout of machinery, and worker training form the backbone of safe practice. MSDS paperwork isn’t just a formality; it sits in break rooms and gets referenced after every spill or incident. Wastewater and runoff control get special attention—polymers can clog water treatment or slip through if not filtered out right. At every step, safety depends on people speaking up when they spot risks, not just waiting for management decrees.
As soon as people realized the sheer absorbency of acrylamide-sodium acrylate copolymers, the uses exploded. Superabsorbent diapers run on these gels, pulling moisture away from skin. In agriculture, they cut watering needs—mixed into soil, they swell after rain then give up water to roots during drought. Medical dressings use them to keep wounds dry without sticking or leaking. In industrial cleanup, crews scatter powdered copolymer to lock down chemical spills. Even hobbyists mix small amounts into floral arrangements or novelty popping toys, chasing the neat effect of liquid vanishing into powder. Sewer repair, civil engineering, and packaging innovation keep pushing the limits, as people look to stop leaks, stabilize soils, or maintain freshness.
Research on acrylamide-sodium acrylate keeps pushing the boundaries, especially as sustainability and recycling climb the agenda. Scientists in labs screen fresh initiators to get cleaner polymers, reducing leftover monomer and side products. Environmental research teams work on breaking down used superabsorbent gels so they don’t hang around in landfills forever. Lately, more teams focus on bio-based acrylates, hoping to replace petroleum-derived monomers. Product designers look at blending with cellulose or chitosan, aiming for strength and biodegradability in the same package. While not every project pans out, the constant push helps catch up to tougher regulations and stricter consumer scrutiny, especially as news cycles spotlight microplastic pollution and end-of-life waste.
No conversation about acrylamide-sodium acrylate feels complete without mentioning its risks. Acrylamide’s reputation follows its polymers—residues in finished product are low, but researchers keep a lookout for chronic effects from workplace exposure or contaminated food. Animal studies raise alarms at higher doses, flagging nervous system problems, reproductive impacts, and cancer connections. Consumer safety groups keep pressure on for transparent reporting and batch testing. Over the years, the industry response moved from vague assurances to strict batch testing, auditing supply chains for monomer levels, and swapping out contaminated feedstocks. Honest reporting and regular monitoring reduce risks, but as old incidents with environmental spills or plant fires show, safety can’t run on autopilot.
Demand for acrylamide-sodium acrylate isn’t dropping anytime soon. More regions look for water-saving agriculture and advanced hygiene products as populations grow and resources shrink. Polymer innovation feeds off that demand—smarter gels, hybrid networks, and easier recycling options. The push for “green” chemistry shapes research programs, driving experiments with enzymes, new monomers, and biodegradable copolymers. Regulation keeps tightening, so future products will need even lower contaminant levels. As waste builds up and consumer scrutiny sharpens, the next breakthroughs won’t just impress in a lab—they’ll get measured by how they shape real-world impact, both in products we use every day and the waste we face down the line.
Few folks chat about acrylamide-sodium acrylate at dinner parties, yet this compound plays a quiet role in so much that touches daily life. What’s often called a “superabsorbent polymer” catches attention mostly for its ability to soak up and lock in water like a sponge on steroids. If you’ve ever changed a baby’s disposable diaper, you’ve already seen what it can do. That mushy gel inside the diaper? That’s the polymer at work, trapping moisture and keeping babies dry.
Flooding never waits for the right moment. I remember a bad storm a few years back where sandbags and traditional barriers just couldn’t keep up. Sandbags are heavy, hard to move, and not very effective after a point. Enter acrylamide-sodium acrylate: this polymer, in the form of small beads or powder, can fill flood barriers or “supersacks.” Just add water, and they puff up, forming a sturdy, water-absorbing wall. Unlike sandbags, these barriers soak up excess water and hold it, making them easier to handle in a crisis. In urban drainage, these polymers get added to soil or used in ditches to help manage stormwater, reducing runoff and cutting down the mess left after storms pass through.
Growing plants in tough places always feels like a gamble against the weather. Too much rain and roots rot. Too little, and crops shrivel. Many farmers have started mixing acrylamide-sodium acrylate into soil—especially in drought-prone regions. The polymer acts like a tiny reservoir, grabbing water during rains and slowly letting go during dry spells. I’ve visited a few community gardens that use this trick: vegetables there stay greener and healthier, even with less frequent watering. Over time, this can mean less strain on water resources, lower bills for irrigation, and fewer failed crops.
It surprised me to learn that the same polymer lands in treatment plants and even basic home water filters. Treatment facilities toss it into tanks during the process of removing particles. The polymer’s charged structure latches onto pollutants, dirt, and other nasty stuff, letting operators filter them out before water hits the tap. For anyone living where clean water can’t be taken for granted, this means an affordable and scalable way to improve supplies. Out in the countryside or on camping trips, portable versions of these polymers show up in compact water-purifying kits.
Useful as acrylamide-sodium acrylate is, it doesn’t come without questions. Its main building blocks, especially acrylamide, can pose health risks if handled carelessly. Regulatory agencies like the EPA keep a close eye on manufacturing and safety standards. I remember chatting with a scientist who stressed how accidents or improper disposal can lead to contamination. Waste management remains one spot ripe for better language and tighter rules. Recycling the used polymer is tough, but cutting down on single-use and setting up proper disposal make a real difference.
Nature’s own cycles can teach us a few things. Industries that use acrylamide-sodium acrylate are testing eco-friendlier tweaks—like blending the polymer with biodegradable additives—without losing the absorption power. On farms, new machinery lets growers bury small amounts right at a plant’s roots, reducing runoff and boosting efficiency. Flood-control products might soon use recycled material collected from old diapers and reuse it for future barriers. People can debate the perfect solution, but the conversation should never lose sight of how much real-world relief these polymers bring and how much smart stewardship matters.
Throw out the fancy labels for a second—acrylamide-sodium acrylate shows up in a lot more places than most folks imagine. This chemical combination pulls a lot of weight in the everyday world. You find it in things like super-absorbent polymers used in diapers, agriculture, and even in some industrial water treatment setups. It works well because it soaks up moisture like a dry sponge at a summer picnic.
I’ve spent years reading up on chemical safety in workplaces and in daily products. Acrylamide on its own gets a lot of concern—scientists point out its ties to cancer in high, long-term exposures. Even the National Toxicology Program keeps it on its 'reasonably anticipated to be a human carcinogen' list. The risk goes up when people deal with the pure chemical dust or fumes. Sodium acrylate packs less risk but isn’t risk-free. It can still cause some serious eye or skin irritation if you aren’t careful.
Regular folks running into acrylamide-sodium acrylate usually get it in the form of gels or powders that don’t pose huge immediate harm. Still, factory workers or labs handling the raw stuff experience a whole different story. Irritated lungs, skin, or eyes aren’t rare if someone skips gloves or a mask. I’ve seen folks underestimate what a careless dust-up can do to your throat or eyes.
Let’s be honest—most ordinary people don’t mess directly with the concentrated chemicals. The risk grows for workers in industries who might be exposed when handling or manufacturing the dry powders or when they’re mixing up big batches. The basics still save the day: gloves, goggles, and a proper mask. Anyone who does it for a living learns these basics pretty fast. A good friend of mine runs safety briefings at a water treatment plant—his team never cuts corners on PPE. Closed shoes, long sleeves, and those blue nitrile gloves are standard gear.
Ventilation matters just as much. Good airflow means less dust hanging in the air. I’ve walked through older factories packed with chemical mist; you notice the lack of fresh air right away. Opening a window isn’t enough. Proper exhaust fans and fume hoods help keep lungs happy.
Safe storage makes a difference too. I remember a story from a plastics warehouse where a half-torn bag led to a week-long headache for a cleaning crew—one small spill, big trouble. Sealed containers, clear labels, and no drinks or snacks nearby set the right routine.
Truth is, in everyday finished products, acrylamide-sodium acrylate doesn’t usually sit on a knife’s edge of safety. The biggest problems come when folks get lazy or complacent during handling and storage. For most consumers, exposure stays low. Watching over kids, pets, or gardens near raw materials still makes sense. The science keeps an eye on potential health effects, but so far, real-world problems come from mishandling, not everyday use.
Keeping people safe always comes down to good habits and clear communication. Better training, regular safety reviews, and respect for warning signs can cut down just about every risk linked to acrylamide-sodium acrylate. I’d love to see stronger rules for labeling and a bigger push to support workers who deal with these chemicals head-on. It pays off—nobody ever said, “I wish I’d worn less safety gear.”
If you spend your days around polymers, you hear people throw out numbers for molecular weight like everybody’s got the same ruler. With acrylamide-sodium acrylate copolymers, there’s a reason for those broad ranges: these things come with more variety than a hardware store’s paint aisle. Most folks working in wastewater treatment, agriculture, or personal care want to know if the molecular weight lands in the sweet spot for their mixes.
Acrylamide-sodium acrylate commonly lands anywhere from about 500,000 to 20 million Daltons. On the low side, you get lighter chains, around half a million. These don’t form thick gels and tend to stay dissolved, which fits certain uses like paper processing or as a soil conditioner where flow and distribution matter. On the high side, you see numbers climbing to 10, 14, even up to 20 million Daltons. These heavier polymers turn water to gel lightning-fast and don’t wash away easily. In my time consulting for a hydroponics project, smaller molecules acted almost like dust in a glass of water, but those heavier chains looked and felt like tapioca gone wild.
It’s tempting to see these numbers and just pick out a size because it shows up cheaper on the supplier’s list. The truth is, polymer size shapes every step after the purchase. High molecular weights trap more water. They also hang around in soils and sediments much longer, which can backfire if you need things to break down quickly after use. At a pulp mill I visited, the difference between a 5-million and a 15-million Dalton copolymer meant either a sticky, immovable mess or a pulp slurry that filtered clean.
Nobody pours chemicals into fields or drains expecting problems, but ignoring molecular weight often invites them. Too high, you get clogging, post-application headaches, and possible buildup in the ecosystem. Too low, the copolymers wash away before doing their job. In personal care, high-weight copolymers deliver that cushiony gel—think luxury face masks—but try rinsing your skin clean, and nobody’s happy with the residue. Polyacrylamide’s reputation in agriculture and water treatment sits squarely on matching the molecular weight to the job, not just the application label.
Lab techs use gel permeation chromatography and light scattering to get precise molecular weights, but most plants order based on suppliers’ ballpark ranges. The “typical” batch most often shows up stamped between 8 and 18 million Daltons for gel-forming uses, lower if the main goal is retention or controlling dust. One study from the Journal of Applied Polymer Science confirmed that even a difference of a few million Daltons can change a polymer’s swelling and retention time radically.
A lot of headaches could fade if industry leaned harder on more detailed spec sheets for these copolymers. Testing a small batch in actual field conditions goes a long way, because lab data can only say so much about how a complex soil or a slurry will respond. Industry folks should also keep an eye on sustainability tech—biodegradable and lower-impact polymers are slowly edging into the space, aimed at solving some of the environmental issues linked to high molecular weight copolymers lingering in soil or water. In the end, staying sharp on the actual molecular weight means cleaner results, fewer surprises, and better fits for whatever the job needs.
You only need to take one careless glance at the back corner of a warehouse—leaky roofs, weird smell, bags stacked under a pipe that’s dripped since last fall—and you start to realize that chemicals like acrylamide-sodium acrylate don’t just “keep” on their own. Stability isn’t a given, it’s earned through daily habits: dry space, cool temps, and respect for the risks. I remember working summers in a paint additive plant where unmarked bags got stashed wherever they fit. Small spills, humidity, and someone’s coffee cup seemed innocent until labels started peeling and product clumped. Suddenly, lost money and safety concerns hit home.
Acrylamide-sodium acrylate’s real enemy sneaks in as moisture. Even in climates that never see rain, a drafty shed or poorly sealed drum pulls water right out of the air. I’ve seen it firsthand—the powder sucks up humidity and turns gummy. This is more than just a storage headache. Once exposed, it loses performance and can start to smell. High humidity even sets the stage for slow breakdown or lumps that make dosing and mixing inaccurate. Keeping this material dry is non-negotiable.
Excess heat never gets along with chemical powders. Overheated warehouses or outdoor containers in July are a gamble. Stored too warm, acrylamide-sodium acrylate speeds toward degradation, and strange side products crop up—none of them friendly. There’s a distinct difference between a storeroom with a simple box fan and one with no airflow. Even a few extra degrees above room temperature can turn a six-month shelf life into a guessing game. I found that out the hard way swapping inventory from a climate-controlled section to a sun-baked shipping dock “just until Friday.” The clumps told the story two days later.
Sealed, original packaging is not just a suggestion from the manufacturer; it’s the lock and key for stability. Cardboard boxes pick up moisture and odors like a sponge, so plastic drums or thick polyethylene liners are worth every cent. Each opened bag begs for resealing—immediately—using moisture-proof tape or tie wraps. Storing opened product in a plastic container with a tight-fitting lid is even better. Fact is, one open bag can spoil the entire pallet if dust drifts or water seeps in.
Clear labeling becomes a lifesaver during audits or emergencies. Sloppy handwriting or half-torn stickers once caused us to mistake acrylamide-sodium acrylate for another white powder. Mix-ups are not rare in messy storage rooms, but a bold mark with a purchase date kept everyone honest.
Most storage failures come down to rushing or skipping steps. Training makes a real difference. At my old plant, weekly checks caught tipped drums and broken seals before moisture spread. Keeping storage areas swept, containers up off concrete, and spill kits within arm’s reach made people take care. Small investments—a pallet rack, a cheap sensor for humidity—save thousands in lost product and prevent headaches when safety inspectors pay a visit.
Few want to think about the extra effort for proper storage, but safer habits and better tools pay off in the long run. Acrylamide-sodium acrylate isn’t a drama queen—just prone to problems if ignored. Give it a cool, dry, tidy spot and keep containers sealed, and most trouble never shows up. Safer products, less waste, and smoother workdays start in that forgotten corner of the warehouse, right where chemical and common sense meet.
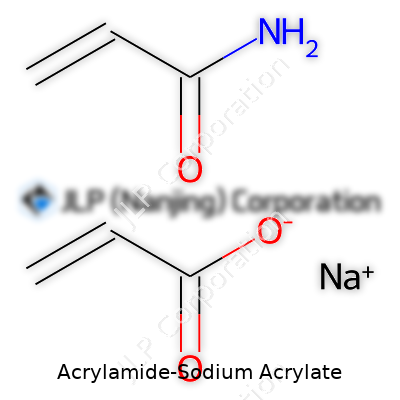
Acrylamide-Sodium Acrylate copolymers show up everywhere from agriculture to the inside of baby diapers. Their rise in the chemical industry didn’t come from some accident but from everyday needs—catching leaks, keeping soil healthy, cleaning water. These two compounds start out simple. Acrylamide brings transparent granules or a powder, barely giving off any smell, dissolving in water with zero fuss. Sodium Acrylate starts as a white powder, reacts as a base, and loves water so much it pulls moisture right out of the air. Mix those two, you get a copolymer with the best of both: the stretch and flexibility of acrylamide, the absorbing power from sodium acrylate.
Water retention stands out. Acrylamide-Sodium Acrylate swells up like a sponge, soaking up hundreds of times its weight. Ever grabbed a baby diaper and been shocked by how heavy it gets? That’s this copolymer doing its job. Early in my lab days, I dropped a pinch into a cup and watched it bloat beyond belief. This makes it a star in drought-prone soils—farmers see bigger yields because their crops don’t dry out between rainfalls.
Clarity also matters, especially in water treatment. Polymers with a higher acrylamide content often go into making water look spotless by grabbing tiny particles floating around. As transparent hydrogels, these polymers show how science shapes what we drink and how communities manage their water.
Now for the chemistry. Acrylamide brings a double bond, giving it stickiness for building long chains with other molecules. Sodium acrylate provides charged carboxyl groups—these attract water and ions, which crank up the polymer’s swelling ability. Once crosslinked, the final product barely dissolves, so it holds together after soaking up liquid, which is a must for everything from cleaning spills to treating wounds.
These carboxyl groups react to salt and pH. Ever tossed table salt on a slippery sidewalk to melt ice? The sodium ions interact with the polymer’s carboxyls, and the copolymer shrinks. Put it in acidic water, and the absorption drops. This sensitivity means engineers have to think about where products get used, especially in places with hard water or pollution.
Sustainability keeps coming up. Most acrylamide is made from petroleum, and leftover acrylamide can be toxic. The industry already faces pressure to clean up manufacturing and manage waste better. A few companies started shifting to bio-based acrylamide, which could help if the price comes down. Still, leftover acrylamide needs careful disposal, and workplace exposure needs close attention—the stuff can harm the nervous system if people breathe in dust or spill it on skin.
Green alternatives take time, but they matter. Farmers and city workers want the absorbing technology, but they also need safe, reliable materials. One smart step: push research into more plant-based chemicals, plus better recycling options. Cleaning up runoff at the plant, using closed-loop systems, and enforcing exposure limits in factories all help control health risks without ditching what works.
Acrylamide-Sodium Acrylate already shapes daily life, from the water people drink to the food they eat. The challenge isn’t just about making it better—it’s about blending chemistry with smart policy, workplace protections, and respect for the environment. Watch for new research and keep asking where these polymers come from and how they fit in a world with tighter safety rules and more pressure on resources. Progress happens when science gets practical and responsible at the same time.
| Names | |
| Preferred IUPAC name | Sodium 2-propenamide-2-carboxylate |
| Other names |
2-Propenamide-2-propenoic acid sodium salt copolymer Acrylamide acrylic acid sodium salt polymer Acrylamide/Acrylic acid sodium salt copolymer Acrylamide/sodium acrylate copolymer Poly(acrylamide-co-sodium acrylate) |
| Pronunciation | /əˈkraɪl.əˌmaɪd-ˌsoʊdiəm əˈkraɪ.leɪt/ |
| Identifiers | |
| CAS Number | 25085-02-3 |
| Beilstein Reference | 110969 |
| ChEBI | CHEBI:61342 |
| ChEMBL | CHEMBL1201577 |
| ChemSpider | 56955654 |
| DrugBank | DB09402 |
| ECHA InfoCard | 50f8f00e-679c-49b4-84e4-f6d031df594a |
| EC Number | 207-173-7 |
| Gmelin Reference | 37724 |
| KEGG | C19130 |
| MeSH | D000198 |
| PubChem CID | 23665797 |
| RTECS number | BR9050000 |
| UNII | 8J87V1O329 |
| UN number | UN3425 |
| CompTox Dashboard (EPA) | DTXSID2023066 |
| Properties | |
| Chemical formula | C5H7NO2Na |
| Molar mass | 207.188 g/mol |
| Appearance | White powder |
| Odor | Odorless |
| Density | 1.322 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -1.0 |
| Vapor pressure | 2.4 mmHg (25°C) |
| Acidity (pKa) | 13.0 |
| Basicity (pKb) | 13.2 |
| Magnetic susceptibility (χ) | −7.4×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.450 |
| Viscosity | 12-18 cps |
| Dipole moment | 5.83 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | Std molar entropy (S⦵298) of Acrylamide-Sodium Acrylate is 191.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -636.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1861 kJ/mol |
| Pharmacology | |
| ATC code | No ATC code |
| Hazards | |
| GHS labelling | GHS07, GHS08, GHS09 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H301, H311, H315, H317, H319, H332, H334, H340, H350, H360, H372 |
| Precautionary statements | P201, P202, P261, P264, P270, P272, P273, P280, P281, P302+P352, P304+P340, P305+P351+P338, P308+P313, P333+P313, P362+P364, P363, P405, P501 |
| NFPA 704 (fire diamond) | 2-2-2-Health |
| Autoignition temperature | > 424°C (795°F) |
| Lethal dose or concentration | LD₅₀ (oral, rat): 124 mg/kg |
| LD50 (median dose) | LD50 (median dose): 107 mg/kg (oral, rat) |
| NIOSH | AS331 |
| PEL (Permissible) | PEL: 0.03 mg/m³ |
| REL (Recommended) | 10 ppm |
| IDLH (Immediate danger) | 30 mg/m3 |
| Related compounds | |
| Related compounds |
Polyacrylamide Acrylamide Sodium acrylate Acrylic acid Acrylonitrile |